The eye has long been recognised as an immuneprivileged organ, whereby immune responses to foreign antigens are suppressed or inhibited. This allows the preservation of normal function within such a highly specialised organ. Immune privilege is maintained by both structural barriers and a complex array of active homeostatic mechanisms. Despite ocular immune privilege, immune-mediated diseases of the eye are very common in many species, for example age-related macular degeneration in people. This is the first of two articles on immune-mediated diseases of the equine eye and will focus on the cornea, with the second article discussing the uveal tract. In both cases, the emphasis will be on the practicalities of treatment and long-term management. A collaborative approach between owner and veterinary surgeon is usually essential from an early stage with immune-mediated disorders, as making owners aware of the potentially progressive and recurrent nature helps to maximise adherence to treatment regimens that are often difficult and frustrating.
There are no treatments licensed for the treatment of immune-mediated ophthalmic disorders in the horse and clinicians are advised to refer to the cascade when selecting appropriate medications. Proprietary ophthalmic formulations of all the topical medications discussed are (intermittently) available in the UK, licensed for either human or animal use. These should always be used in place of non-ophthalmic preparations, which can cause severe damage to the eye. Some compounded preparations are now also available as well. Sustained release ciclosporin A implants require a special treatment certificate for importation into the UK. Many preparations are also prohibited in competition horses.
Immune-mediated keratopathies
The immune-mediated keratopathies (IMMKs) are a group of poorly defined, non-ulcerative, inflammatory disorders of the cornea, where the aetiopathogenesis involves an abnormal, typically upregulated immunoresponsiveness of the cornea (Matthews 2000). Historically, their classification has not been straightforward or consistent (particularly geographically between the USA and the UK), causing understandable confusion among veterinary surgeons. This section presents four categories of immune-mediated corneal disorders aimed at practitioners in the UK, based on current understanding: the three IMMKs, eosinophilic keratitis, other superficial IMMKs (including viral keratitis), and miscellaneous disorders.
Category 1: the three IMMKs
These are defined based on their location within the cornea (chronic superficial IMMK; chronic deep/mid-stromal IMMK; and endotheliitis/endothelial IMMK) (Brooks et al, 2017). The hallmark findings for all three IMMKs are: corneal oedema – this may be focal or generalised and varying in opacity (usually much more so than the oedema of glaucoma for example); corneal cellular infiltration (and occasionally associated bullae formation, pigmentation, mineralisation and fibrosis), which appear as grey/yellow/white/green discolouration of the cornea; corneal neovascularisation – extending from the limbus for varying lengths and at varying depths; and an absence of uveitis or overt ocular pain (in comparison with the likes of infectious ulcerative keratitis or uveitis), unless secondary complications have occurred. Most cases are progressive, and it is important that subtle signs of IMMK are detected during prepurchase examination.
- Chronic superficial IMMK (Figure 1) has an insidious onset and is usually steadily progressive without overt eye pain. The condition often starts dorsally on the cornea and the apposing region of palpebral conjunctiva may be inflamed. Prominent subepithelial arborising neovascularisation is seen arising from the limbus, with perivascular oedema a common feature. White to yellow stromal cellular infiltrate may be present but is usually much less pronounced than for mid-stromal IMMK.
- Chronic deep/mid-stromal IMMK (Figures 2 and 3) is typically episodic in nature (usually occurring two or three times per year but sometimes intervals between active phases can be much longer and the disease might appear to have resolved). In the active phase of the disease there is an extensive and dense mid-deep stromal oedema and fibrovascular response. Lacunae of green-/orange-tinged fluid may collect within the mid-stromal cornea. Pain is rarely a feature despite the very abnormal appearance, although rupture of subepithelial bullae and subsequent painful ulceration can occur. Subepithelial calcium deposition may be seen as white opacities beneath the epithelium. During the quiescent phases there is variable corneal oedema, fibrosis and neovascularisation.
- Endotheliitis/endothelial IMMK (Figures 4 and 5) appears to be mostly acute in onset in the UK and is characterised by diffuse central oedema that often extends throughout the entire cornea but can be restricted to clearly delineated regions. Glaucoma is an important differential. Hydrops can be a feature. Prominent arborising vessels extend into the deep stroma and Descemet's membrane, with cellular infiltration commonly seen around the terminal vessels. Ocular discomfort is extremely rare.
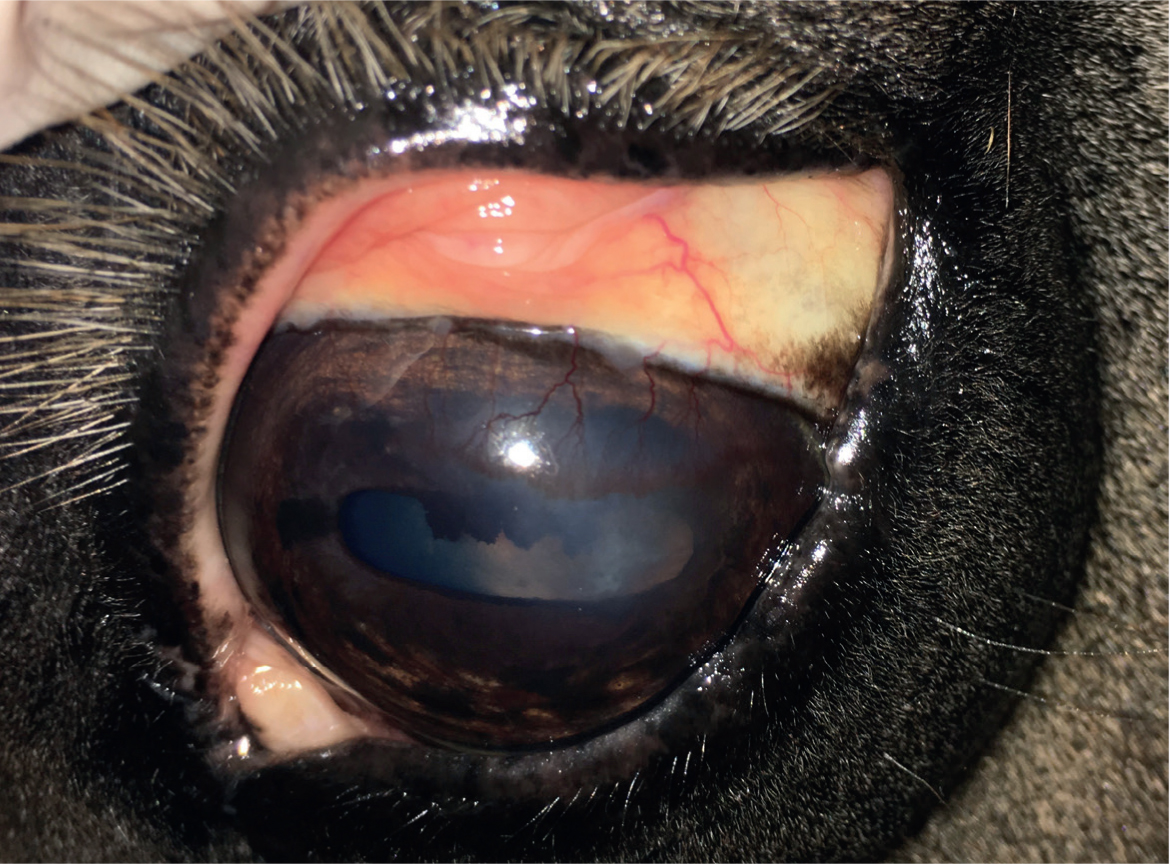
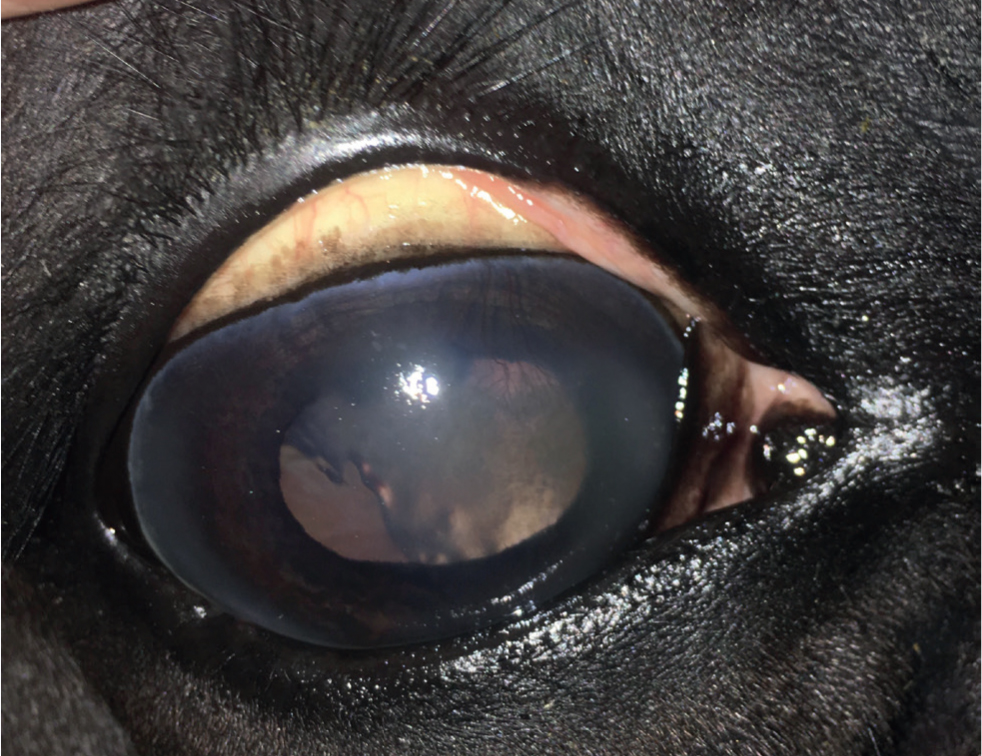
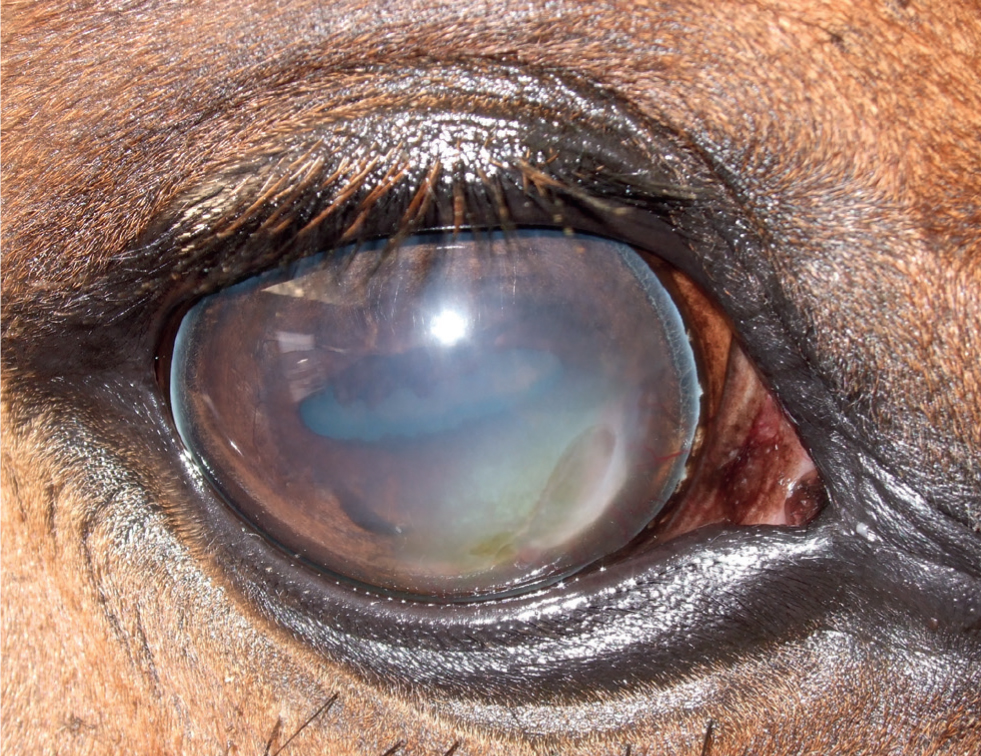

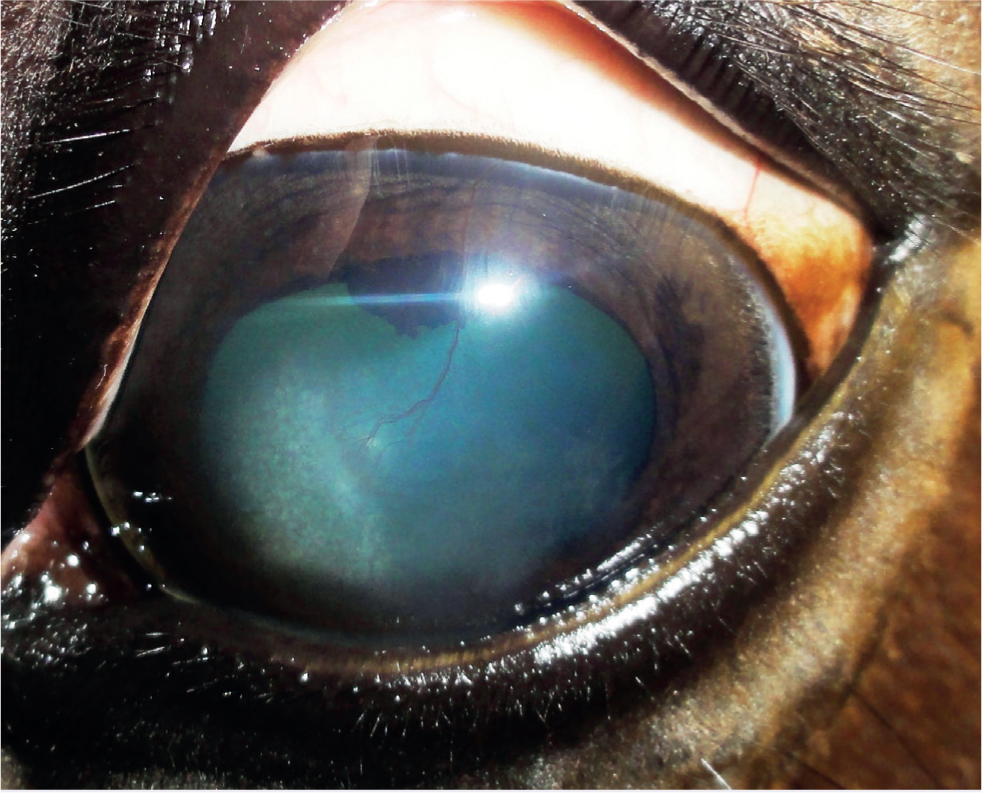
The long-term impact and clinical significance of IMMK is variable and depends on the stage and type of disease. Ocular discomfort caused directly by the IMMK is usually only mild. Secondary corneal ulceration, subsequent infection and uveitis cause more severe discomfort. This can become recurrent and is usually slower to resolve than in a healthy cornea. Therefore, pain from recurrent secondary complications is a serious welfare concern in many cases, potentially resulting in enucleation. The varying amounts of corneal oedema is obviously an impediment to vision, although no other damage to the visual pathway occurs directly from IMMK. Complete diffuse oedema causing blindness is rare and most horses appear able to visually cope well with the IMMKs, especially where the condition is unilateral. Interestingly, a report suggested that malignant transformation of T lymphocytes associated with chronic IMMK might have been the source of corneal B-cell lymphoma in a horse (Vallone et al, 2016).
Aetiology
The development of most autoimmune disease is a combination of genetic and environmental factors. Given the variety of presentations and response to treatment, it has been suggested that IMMK should be considered a syndrome, rather than a specific diagnosis, with completely different disease processes occurring in different horses (Brooks et al, 2017). A long-suggested pathogenesis is that the cornea recognises an autoantigen presented locally (molecular mimicry) or a foreign protein (including microbial epitopes), which bypasses immune privilege. This triggers an immunoin-flammatory response in the cornea, which itself drives further loss of immune privilege and continued deterioration (Matthews and Gilger, 2010). An infectious agent may be the inciting or perpetuating cause (or both), although no specific trigger has ever been identified. The pathogenesis of superficial stromal IMMK involves cell-mediated inflammation governed by both cytotoxic and T helper cells (Pate et al, 2012). A more recent study suggested that some horses with IMMK produce autoantibodies against a corneal protein (maspin), however the authors were clear that this was not conclusive evidence that IMMK is an autoimmune disease (Braus et al, 2017). In-vivo confocal microscopy demonstrated that a common distinguishing feature of epithelial, superficial and mid-stromal forms of IMMK is a dense accumulation of dendritic cells in the epithelial basement membrane and immediate subepithelial stroma, whereas cellular changes in the endothelial form were largely confined to the endothelium (Ledbetter and Irby, 2020). The authors suggested that this may represent a separate aetiopathogenesis for endothelial vs stromal forms of IMMK, although they acknowledge that many of the changes could simply reflect the result of chronic stromal inflammation. Overall, the aetiology of IMMK must still be described as unknown.
Treatment
Initial investigation and decision making usually focuses on elimination of other important causes of corneal oedema, neovascularisation and cellular infiltration – in particular trauma, glaucoma, equine recurrent uveiitis, stromal abscessation and ulcerative keratitis. A complete ophthalmic examination, including fluorescein staining of the cornea and measurement of intraocular pressure, should be carried out. Initial treatment is directed toward any of these conditions if present and will not be discussed further here.
Failure of any abnormalities to respond appropriately to standard treatments should raise suspicion of an underlying IMMK. A definitive diagnostic test for IMMK is not available, but further investigation by ocular cytology and culture should always be attempted to rule out infectious agents or eosinophilic keratitis (see below), before focusing therapy towards IMMK (Matthews and Gilger, 2010). This is particularly important for stromal abscessation and fungal infection, where immunosuppressive therapy may precipitate rapid deterioration. A diagnosis of IMMK can be made if there is a progressive or chronic (>3 months' duration) nonulcerative or recurring corneal opacity with mild to moderate signs of cellular infiltrate, corneal vascularisation and corneal oedema (Gilger et al, 2005; Matthews and Gilger, 2010). The obvious delay that this entails in reaching a definitive diagnosis is frustrating, as early intervention is thought to be important (Brooks et al, 2017).
Topical anti-inflammatory, immunosuppressive and im-munomodulatory therapy is initially indicated for all types of IMMK. Further treatment options including sustained release im-plants, superficial keratectomy and stem cell therapy can then be considered. As data (albeit still very limited) describing the efficacy of various treatments have emerged, there is increasing evidence to recommend certain treatment options depending on the classification of the IMMK.
- Corticosteroids have wide-ranging anti-inflammatory and immunosuppressive effects that make them an ideal choice for treatment of IMMK. Their ability to inhibit angiogenesis is also particularly important, as this neovascularisation contributes to the loss of immune privilege within the cornea that helps to propagate IMMK and corneal deterioration. Corticosteroids are generally contraindicated where corneal ulceration is present. Combined 0.1% dexamethasone/polymyxin B/neomycin ointment/solution (Maxitrol; Novartis Pharmaceuticals UK) and a 1% prednisolone acetate (Predforte; Allergan) are available. Both are usually applied three to four times daily if possible.
- Ciclosporin A is an immunosuppressive in the calcineurin inhibitor class that selectively inhibits T cell proliferation and activity. This helps to minimise the non-specific immunosuppressive effects on other cell types, including neutrophils and macrophages, that are seen with corticosteroids. Unlike corticosteroids, ciclosporin A can be used alongside antimicrobial treatments for ulceration before re-epithelialisation occurs where there is suspicion of underlying IMMK (Gratzek et al, 1995). Ciclosporin A is available in the UK as a 2 mg/g ophthalmic ointment (Optimmune; MSD Animal Health) that is licensed for use in dogs for the treatment of various ocular autoimmune disorders. It is also licensed to augment topical steroid use. In the horse, ciclosporin A is usually applied twice daily initially and costs considerably more than corticosteroid. Penetration to the corneal stroma is good, but it is considered unlikely to reach the endothelium. At the time of writing, ciclosporin A was permitted for ophthalmic use in horses competing under Fédération Équestre Internationale rules, as long as the appropriate veterinary form had been completed. Another calcineurin inhibitor, tacrolimus, is also increasingly being used in the horse. However, there is no equine-specific evidence available to justify its use. In dogs, tacrolimus appears as effective as ciclosporin A and has been effective in cases refractory to ciclosporin A (Hendrix et al, 2011). A compounded formulation is available.
- Topical non-steroidal anti-inflammatory drugs (NSAIDs). These can be used for cases where corticosteroids are contraindicated as a result of corneal ulceration and ciclosporin A is not an option. They are generally considered to be much less effective and are not generally recommended for IMMK. Topical bromfenac has been reported as a useful treatment for endotheliitis in the USA (Matthews and Gilger, 2010). Systemic NSAID use is not usually indicated, unless secondary complications are present.
- Doxycycline has specific anti-inflammatory and immunosuppressive effects that target T-cell mediated inflammation and it has also been suggested to have antiangiogenic effects. At the time of writing, a topical ophthalmic preparation of doxycycline was not available and the use of systemic formulations is considered inappropriate based on the potential for adverse effects and development of resistance. Together with the absence of any published evidence of efficacy and in line with current advice on responsible use of antimicrobials, the use of doxycycline cannot be recommended for treating IMMK and should be considered irresponsible.
The response of the IMMKs to either topical ciclosporin or corticosteroid appears to vary greatly; many cases seem responsive initially (although rarely to the point of complete resolution), but-Fin time become refractory with increased relapses and chronic changes within the cornea. The reasons for this are unknown, but changes in geographical location and chronicity before referral have been suggested (Matthews and Gilger, 2010). Owners must be made aware that treatment will almost certainly be lifelong with sporadic intermittent recrudescence in most cases. Overall, as the IMMKs are usually non-painful and very effective control can be achieved for long periods of time, therapy should always be attempted before discussing enucleation.
Initially, the combined use of both corticosteroids and ciclosporin A at their full therapeutic doses (i.e. applying each two to three times daily with a short interval of minutes between treatments) is recommended for treatment of chronic superficial and deep-stromal IMMK, to attempt to bring it under control as rapidly as possible (Brooks et al et al, 2017). Sole therapy with corticosteroid can be used where finances are more limited, as long as no ulceration is present, and is the recommended first-line treatment for endotheliitis.
If initial therapy is successful (defined here as IMMK improved to the point where it is not considered significant to the horse, not complete resolution) then long-term management strategies can be considered. Medications should be slowly tapered to find the minimum effective dose and combination. This can be difficult as the severity of IMMKs often wax and wane, and successful management relies on owner adherence to the plan and regular veterinary examinations.
Topical medication is certainly the cheapest option if corticosteroids are the sole therapy. Long-term administration of corticosteroid to the eye can potentiate infection, delay healing of ulceration and increase the chance of calcific band keratopathy (which can be a consequence of IMMK and confuses the clinical picture). Where ciclosporin A is used there are generally fewer adverse effects reported, but the cumulative costs can quickly become similar to those associated with more advanced treatments. Overall, however, excellent control can be achieved for several years in many horses using combinations of topical ciclosporin A and corticosteroid. Key factors are the compliance of owner and horse with administration of medication one to four times daily; a very observant owner; regular veterinary ophthalmic examination; and a certain amount of luck regarding the IMMK you are dealing with. All of this should be discussed and understood before treatment begins and a realistic plan formulated between the veterinary surgeon and owner. If topical therapy is unsuccessful, IMMK becomes refractory to treatment or compliance issues mean that therapy must be discontinued, then further treatment options are as follows:
- Implantation of a sustained-release ciclosporin A device. The use of an episcleral silicone matrix ciclosporin A delivery device to control IMMK has been reported and is currently the recommended treatment (Braus et al, 2017; Gilger et al, 2014). An initial study showed treatment success in nine eyes with superficial keratitis and five eyes with endotheliitis, and treatment failure in three cases of mid-stromal IMMK (Gilger et al, 2014). However, of the nine cases of superficial IMMK, two also underwent superficial keratectomy at the time the implant was placed and two required long-term topical ciclosporin A application (albeit only once daily) and of the five cases of endotheliitis, three of the five continued topical treatment with bromfenac. Reducing the frequency at which the medicine needs to be applied can be hugely important to maintaining treatment compliance and, anecdotally, the implants deliver excellent control in many cases. Evidence of some positive response to topical ciclosporin A is a prerequisite for implant placement. Episcleral implants can be placed in the standing horse, in contrast to the suprachoroidal implants used to treat equine recurrent uveiitis. Suprachoroidal implants have been shown to improve cases of IMMK (Tóth et al, 2011); however, the location is aimed at delivering medications to the posterior segment and drug delivery is thought to be superior with episcleral positioning.
- Superficial keratectomy. Superficial keratectomy with or without a conjunctival graft have been used in cases of chronic superficial and mid-stromal IMMK that are unresponsive or refractory to medical therapy (Matthews and Gilger, 2010). Good results have been reported for the treatment of superficial IMMK only (Gilger et al, 2005; Pate, 2010). The use of superficial keratectomy and Gunderson inlay flap surgery to improve corneal oedema associated with endothelial dysfunction and inflammation has recently been reported in four horses, however the aetiology of the cases appeared mixed and none were defined as a primary IMMK (Scherrer et al, 2017). Surgery is generally reserved for those cases refractory to all medical therapy.
- Sub-conjunctival injection of autologous bone marrow-derived mesenchymal stem cells. Stem cells have a reported ability to reduce corneal inflammation and opacification, and thus there has been interest in their use in IMMK. A recent report involving four horses with IMMK that were poorly responsive to medical therapy, showed some improvement in three of the four cases (Davis et al, 2019). Overall though, the evidence is unconvincing at present and more evidence is required before the treatment can be recommended.
Category 2: eosinophilic keratitis
Eosinophilic keratitis has long been identified in horses, but the incidence appears to be increasing. It is hypothesised that it represents a delayed hypersensitivity reaction to parasitic or environmental allergens that may originate in the conjunctiva, although ultimately the pathogenesis that drives the eosinophil recruitment to the cornea remains unknown (Lassaline-Utter et al, 2014; Brooks et al, 2017; González-Medina, 2019). In human corneal epithelial cells, the release of eosinophil granule proteins results in inflammation, mast cell degranulation, inhibition of wound healing, neutrophil activation and detrimental effects on corneal epithelial cell viability and morphology (Trocmé et al, 1997).
Eosinophilic keratitis is often bilateral (compared with the IM-MKs, which are typically unilateral) and shows seasonal occurrence in the northern hemisphere, with increased incidence in summer and autumn. Eosinophilic keratitis generally appears to be more painful than the IMMKs and the initial presenting clinical signs include blepharospasm, epiphora, conjunctival hyperaemia and secondary infectious corneal ulceration (Brooks et al, 2017). Corneal neovascularisation is variable, but can be pronounced. Corneal cytology usually provides a definitive diagnosis and the use of a cytology brush is recommended over a swab for sampling. A review described three distinctive clinical presentations seen in horses in the UK (González-Medina, 2019):
- Localised non-progressive form – minimal corneal involvement and ocular pain with lesions mostly around the third eyelid margin (can be difficult to visualise here) that are commonly reported as non-healing ulcers. Sometimes a small, white/cream plaque is seen overlying the area that is easily detached.
- Progressive extensive form (Figure 6): marked ocular pain with extensive cornea lesions that progress rapidly from the periphery towards the central cornea. White/cream plaque formation and secondary infectious ulceration are more common than with the non-progressive form. Involvement of the deeper stromal layers also occurs and can make treatment more difficult.
- A final, rare form is the superficial multifocal form, in which multiple caseous yellow foci can be spread over the corneal surface, mostly in perilimbar locations. Moderate to severe corneal oedema is usually present.
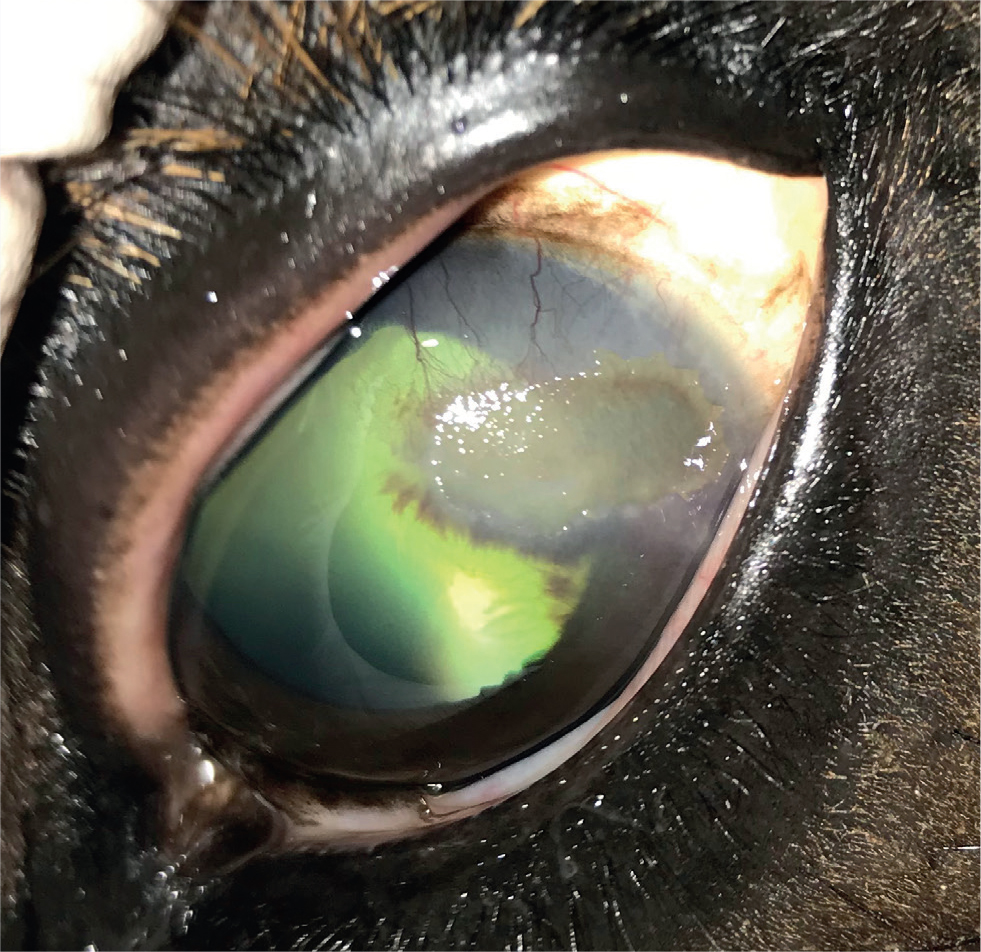
Treatment
While it is generally agreed that standard treatment of any associated infected corneal ulceration, conjunctivitis and uveitis should be carried out where required (topical antimicrobials, antifungals, mydriatics and systemic NSAIDs), there is still some debate over the best way to specifically treat eosinophilic keratitis (González-Medina, 2019). Specific therapy is generally based on the use of topical immunosuppressive therapy, ideally using corticosteroids (although the use of ciclosporin A is also reported), and gentle de-bridement of any caseous material (Brooks et al, 2017; González-Medina, 2019). The use of topical corticosteroids is generally contraindicated where infected, ulcerated areas of cornea are present and making this differentiation can be very difficult. Eosinophilic plaques typically stain weakly with fluorescein. Concurrent treatment with oral cetirizine (0.4 mg/kg twice daily) has been shown to reduce recurrence of eosinophilic keratitis and is recommended throughout the treatment period (Lassaline-Utter et al, 2014). This article also suggested that oral dexamethasone may reduce the duration of treatment. Topical NSAIDs have been reported to in-crease the severity of eosinophilic keratitis and should be avoided (McEwan, 1990). Based on their success in humans, there is a belief among some ophthalmologists that superficial keratectomy should be carried out soon after diagnosis with eosinophilic keratitis (McEwan, 1990). Diamond burr debridement of the localised non-progressive form may accelerate healing (Brooks et al, 2017). The use of topical mast-cell stabilisers (such as N-acetyl aspartyl glutamic acid and iodoxamine) has been suggested, yet their pharmacokinetics and efficacy in horses has never been studied (Brooks et al, 2017). The prognosis for eosinophilic keratitis is generally good where treatment can be applied, although the time to resolution is very protracted (one study reported an average time of 3.7 months with a range of 1–12 months) (Lassaline-Utter et al, 2014). The use of sub-palpebral lavage devices can be useful in these cases, where treatment is protracted, but potentially not permanent.
Category 3: other superficial immune-mediated keratopathies
These are a group of poorly described immune mediated diseases affecting only the most superficial part of the cornea. They are often frustratingly difficult to definitively diagnose as a result of their similarities and are described together here to try and highlight the differences. Cytology should be carried out before attempting treatment, as epithelial non-ulcerative keratomycosis can appear similarly.
- Epithelial keratopathy (previously often referred to as epithelial IMMK) is a condition associated with feint, diffuse, usually central, superficial corneal opacity. Unlike the other forms of IMMK, it is associated with mild ocular discomfort and slight blepharospasm (and occasionally chemosis and conjunctival hyperaemia). Neovascularisation is rare. On close inspection, the opacity is often made up of multiple small clumps of epithelial cells that retain fluorescein stain between the clumps. Epithelial IMMK usually responds well to topical corticosteroids.
- Superficial punctate keratopathy has also been described and is characterised by multifocal punctate opacities affecting the most superficial layers of the subepithelial stroma only (i.e. the surface epithelium appears normal and smooth, with no stain uptake, unlike the true epithelial keratopathies) (Brooks et al, 2017). The cause is unknown and treatment is often ineffective, although topical corticosteroids can result in transient improvement.
- Putative viral keratitis. This is a condition that is familiar to many clinicians, with herpes viruses (particularly EHV-2) most frequently implicated as the cause. Corneal disease associated with herpes simplex virus is recognised in humans, but there is currently no evidence confirming the role of a viral agent in primary keratopathy in the horse, hence the term putative. This certainly does not exclude its existence although and the widespread incidence of suspect viruses will always make it difficult to prove causality. To add further confusion, it is speculated that some IMMK may be associated with chronic reactivation of latent herpesviruses or retention of virus-derived epitopes within the corneal stroma (González-Medina, 2015). Putative viral keratitis is reported to present as an acutely painful condition (often triggering secondary uveitis) that is associated with multifocal punctate opacities caused by ulceration (fluorescein positive) and erosion (Rose Bengal positive) of the cornea (Figure 7) (González-Medina, 2015; Brooks et al, 2017). Cases of putative viral keratitis are reported to improve rapidly following topical administration of topical antivirals, including idoxuridine or a combination of aciclovir and ciclosporin A (Matthews and Handscombe, 1983; McMullen, 2004). In humans, aciclovir is recommended over idoxuridine when treating herpetic keratitis, because of improved efficacy and reduced adverse effects on the cornea (Balderson et al, 2015; Tsatsos et al, 2016). Proprietary ophthalmic ointment preparations of aciclovir were unavailable at the time of writing in the UK, however, ganciclovir is available and appears to be an effective alternative.
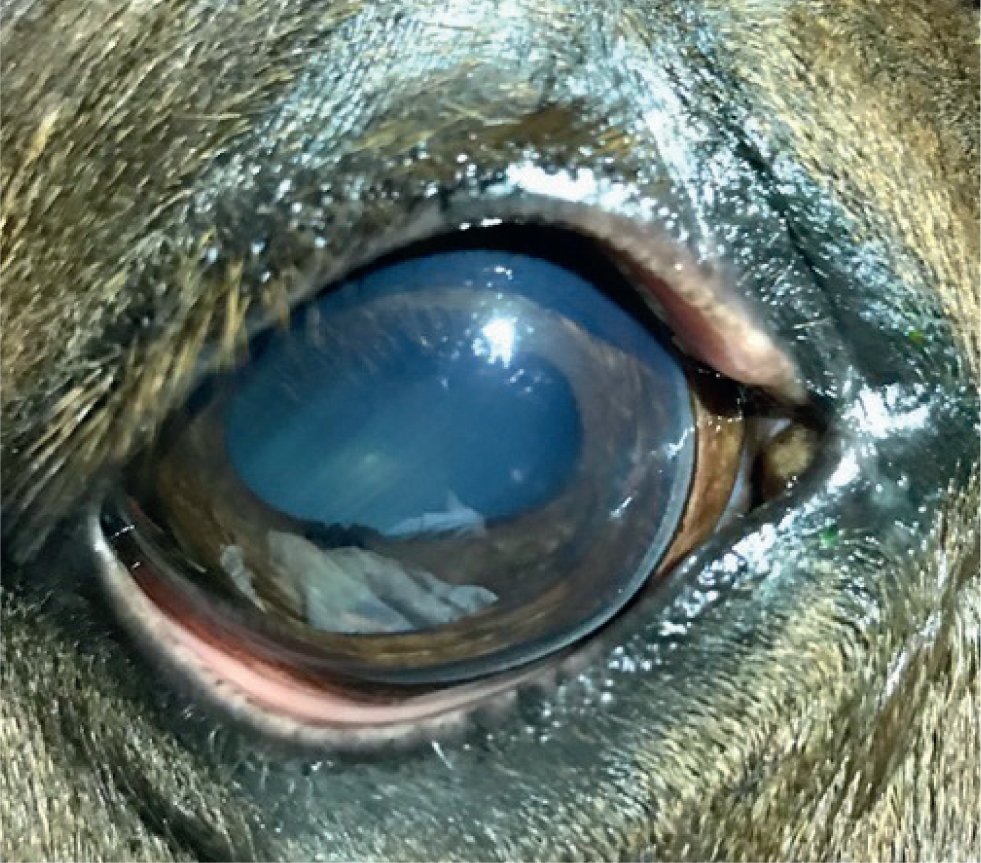
Category 4: miscellaneous
Keratoconjunctivitis sicca is a very rare condition of horses and is usually secondary to facial nerve paralysis or damage to the upper eyelid. Immune-mediated keratoconjunctivitis sicca caused by primary lacrimal adenitis (as is common in dogs) has not been demonstrated in horses; however, there are reports of therapeutic response to immune-suppressing treatment in horses with dry-eye and no evidence of nerve or eyelid damage (Reilly and Beech, 1994; Giuliano, 2017; Mackenzie et al, 2017). Clinical signs vary and include blepharospasm, mucopurulent ocular discharge, keratitis and a lacklustre appearance of the cornea. Clinical signs often improve with treatment, as nearly all treatments lubricate the eye, before recurring on cessation of therapy. Keratoconjunctivitis sicca is a differential diagnosis for immune-mediated disorders of the cornea. Diagnosis is based on clinical suspicion and Schirmer tear test measurements of ≤10–15 mm/min (Giuliano, 2017). Topical tacrolimus or ciclosporin A are potential treatments and successful use of episcleral ciclosporin A implants has been reported (Mackenzie et al, 2017).
Several other recognised non-ulcerative keratopathies of horses may also be immune-mediated. These include pannus, episclerokeratitis, pigmentary keratitis and heterochromic iridocyclitis with secondary keratitis (Matthews and Gilger, 2010; Pinto et al, 2015). These conditions all occur rarely and sporadically, and as such are not discussed further in this article.
Conclusions
Overall, there are several immune-mediated disorders of the equine cornea that practitioners may encounter. The distinction between these conditions can be incredibly difficult, particularly as secondary complications often confuse the picture and are usually far more overt, making selecting the appropriate treatment difficult. Early input from specialist clinicians can be particularly useful in formulating long-term treatment plans that are suitable for both owner and horse, as well as in discussing potential surgical options before available funds are depleted.
KEY POINTS
- There are several immune-mediated disorders of the cornea that clinicians should be aware of.
- Most are non-painful but can result in painful secondary disorders such as uveitis and ulceration.
- Oedema, cellular infiltration and neovascularisation are prominent features.
- Cytology is very important to rule out differentials.
- Treatment is usually long-term to permanent and involves the use of topical immunosuppressants/immunemodulators, implants and (rarely) surgery.
- Early input from specialist clinicians can help to give the best long-term outcome.


