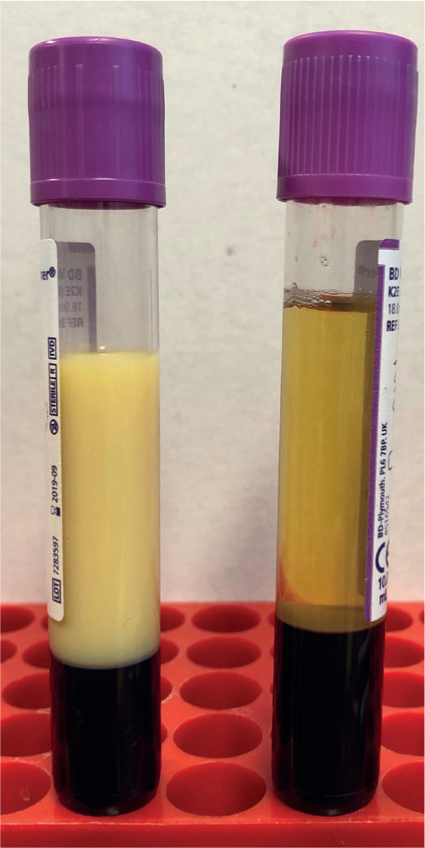
Disorders of lipid metabolism are characterised by an increase in levels of circulating lipids, specifically triglycerides, which occurs as a result of a negative energy balance. This negative energy balance arises from a mismatch between the body's needs (for example severe inflammation, gestation and lactation) and its intake (for example anorexia and gastric reflux). Stressors like transportation can precipitate this condition. The clinical signs of lipids disorders include inappetance and dullness, but might not be very specific. The excessive circulation of lipids can often be identified by the opalescence of the serum (Figure 1). While mild to moderate increases in circulating lipid concentrations may have relatively mild clinical consequences, severe increases can result in excessive fat deposition in the liver and the kidneys, impairing their function and often leading to serious complications and even death.
Lipid metabolism
In horses, lipids are absorbed from the gastrointestinal tract in the form of fatty acids. Short-chain fatty acids bind to albumin and travel through the portal circulation, but most fatty acids undergo transformation into phospholipids and triglycerides in gastrointestinal epithelial cells (van der Kolk et al, 1995). They are then transported in the portal circulation or incorporated as chylomicrons and transported through the lymphatic circulation. Horses are hindgut fermenters, which gives them the ability to change from monogastric glucose-oriented metabolism to a ruminant fatty acid-oriented metabolism (Argenzio and Hintz, 1972; Naylor et al, 1980).
In the liver, fatty acids have two metabolic pathways which are dictated by the body's energy demands. They can be esterified with glycerol to form triglycerides and be exported or stored locally. They can also be oxidised into acetyl coenzyme-A and be used to provide energy. As triglycerides, fatty acids combine with cholesterol, proteins and carbohydrates to form very-low-density lipoproteins (rich in triglycerides) or high-density lipoproteins (richer in phospholipids and proteins) released into the bloodstream. Those lipoproteins are then used as source of triglycerides for adipocytes and all cells containing lipoprotein-lipase. In those cells, fatty acids enter the Krebs cycle after transformation into acetyl coenzyme-A. Alternatively, they can be transformed into steroids, cholesterol, ketones or other fatty acids (van der Kolk et al, 1995). Unlike other herbivores, the formation of ketones is very limited in horses (Rose and Sampson, 1982).
The major regulator of lipid metabolism is energy demand. Positive energy balance favours lipogenesis and negative energy balance favours lipolysis and lipid mobilisation. After a meal, glucose absorption results in insulin secretion into the bloodstream. Insulin increases glucose uptake by sensitive tissues, and blood glucose is maintained in a narrow range. Absorbed glucose is used as a source of energy, stored as glycogen in muscles or the liver or stored in fat in adipocytes. As an anabolic hormone, insulin also inhibits the hormone-sensitive lipase which inhibits the breakdown of triglycerides and stimulates the lipoprotein-lipase that increase the uptake of triglycerides by peripheral tissues (Suagee et al, 2010). When glucose concentration decreases, because of limited food availability or high metabolic needs, insulin secretion decreases and glucagon secretion increases. This shift in hormonal balance prompts the use of lipids as an energy source, triggering lipolysis (Naylor et al, 1980; Freestone et al, 1991; Frank et al, 2002). Glycerol and fatty acids can be metabolised locally to provide energy, either directly or through beta-oxidation (Hughes et al, 2004). In some cases, excessive lipolysis leads to the conversion of fatty acids into very-low-density lipoproteins released into the bloodstream. Those fatty acids and very-low-density lipoproteins are taken up by the liver to be esterified into triglycerides to recirculate in the systemic circulation or to be oxidised locally to provide energy (Naylor et al, 1980; Freestone et al, 1991).
This metabolic shift also increases catecholamines and glucocorticoids secretion which antagonise insulin actions and upregulate hormone-sensitive lipase activity resulting in further lipolysis (van der Kolk et al, 1995; Breidenbach et al, 1999; Lavery and Glover, 2000; Langin, 2006). Limited food availability or high metabolic needs also increase catecholamines and glucocorticoid secretion which antagonise insulin action, reducing further the inhibition of the hormone-sensitive lipase resulting in exacerbated lipolysis. Certain factors such as breed, age or endocrine background can predispose horses to impaired lipid metabolism and unregulated lipolysis. For example, ponies are more efficient at releasing very-low-density lipoproteins into the circulation and are more susceptible to catecholamines and glucocorticoids, leading to higher mobilisation of fatty acids and triglycerides in cases of negative energy balance (Breidenbach et al, 1999).
Hyperlipaemia
Hyperlipemia is a syndrome characterised by serum opalescence associated with circulating triglyceride concentration above 5.5 mmol/litre (Gay et al, 1978; McKenzie, 2011). This condition can be life-threatening because of fat accumulation in the liver (Murray, 1985). Hyperlipidaemia and hypertriglyceridaemia are milder forms of the condition, with circulating triglyceride concentrations below 5.5 mmol/litre and 1.1 mmol/litre respectively (Waitt and Cebra, 2009). With these milder forms, serum opalescence and fat infiltration of the liver are inconsistent (Dunkel and McKenzie, 2003; McKenzie, 2011).
Epidemiology
Hyperlipaemia is more common in ponies, with an incidence as high as 5%, but not as commonly diagnosed in horses (Jeffcott and Field, 1985a; 1985b; Watson et al, 1992a). There is no proven genetic predisposition, although the majority of reported cases involve ponies from native British breeds (Jeffcott and Field, 1985a; 1985b; Watson et al, 1992a). Donkeys are also commonly affected by the condition (Moore et al, 1994; Durham, 2006; Waitt and Cebra, 2009).
The typical profile of hyperlipaemia is an obese middle-aged female equine in late gestation, early lactation or experiencing colitis (Jeffcott and Field, 1985b; Watson et al, 1993; Waitt and Cebra, 2009). Despite the association with gestation, there is no seasonal predisposition for the disease (Watson et al, 1992a). Table 1 shows predisposing and triggering factors associated with the development of hyperlipaemia.
Table 1. Predisposing and triggering factors associated with the development of hyperlipaemia
| Predisposing factors | PonyDonkeyObesityTissue insulin resistanceAge |
| Triggering factors | Systemic inflammationGastrointestinal disease (colic, enterocolitis)LactationGestationTransportDental diseaseNeoplasiaStress |
Pathogenesis
More than half of the ponies diagnosed with hyperlipaemia had been exposed to some form of stress within the 4 weeks preceding the diagnosis (Jeffcott and Field, 1985b). Stress triggers the release of catecholamines and glucocorticoids which antagonise the effect of insulin and promote uncontrolled lipolysis (Jeffcott and Field, 1985a). This effect is particularly severe in obese animals including donkeys, where insulin sensitivity is already compromised (Hughes et al, 2004; McKenzie, 2011).
Commonly reported stressors include transport, gestation, lactation and systemic disease (mainly colic or enterocolitis). In all of these cases, there is a mismatch between an increased energy demand and decreased caloric intake, leading to a state of negative energy balance (Watson et al, 1992a; 1993; Hughes et al, 2004; Conwell, 2010). Lipolysis leads to a significant increase in circulating fatty acids, cholesterol, triglycerides and very-low-density lipoproteins (Watson et al, 1992a; 1993; Tóth et al, 2018). Since horses have a poorly developed ketone pathway, higher fatty acid concentrations stimulate the liver to synthesise more triglycerides and release more very-low-density lipoproteins (McKenzie, 2011). This increased release of very-low-density lipoproteins stimulates the activity of lipoprotein lipases and hepatic lipases, resulting in fatty infiltration of various organs including the liver (Watson et al, 1993). It is important to note that the primary issue is not with hepatic lipid catabolism, but rather with the excessive production of very-low-density lipoproteins, which leads to hyperlipidaemia and liver fatty infiltration.
Clinical presentation
Clinical signs (Table 2) are often overlooked because of their vague nature (Dunkel et al, 2014). Initially, these signs may include mild behaviour changes, which are usually attributed to the primary disorder responsible for hyperlipidaemia (Gay et al, 1978; Murray et al, 1985). Clinical signs can become more obvious and include dysfunctions of the gastrointestinal tract and the nervous system; however, at that stage the disease might already be irreversible (Jeffcott and Field, 1985b; Mogg and Palmer, 1995; Hughes et al, 2004). In the final stages of the disease, about a third of the animals will exhibit clinical signs indicative of central nervous system dysfunction, such as convulsion and mania (Gay et al, 1978; Murray, 1985; Moore et al, 1994). Unlike other liver diseases, icterus is a rare feature of hyperlipaemia (Hughes et al, 2004).
Table 2. Clinical signs of equine hyperlipaemia and their reported frequency
| Disease stage | Clinical signs | Frequency in the reported studies |
|---|---|---|
| Early stage | Depression and drowsinessInappetence and anorexiaAdipsiaNoneWeight lossIleus | 95%87%64%43%43%Inconsistent |
| Intermediate stage | FeverColicVentral and limb oedemaDiarrhoeaMuscle fasciculationsTachycardiaIncoordinationIcterusTachypnoea | 66%64%53%47%46%33%InconsistentInconsistentInconsistent but marked |
| Terminal stage | Central nervous system signs: convulsions, nystagmus, maniaDeath | 33%22–80% |
Diagnosis
Identifying hyperlipaemia through clinical signs can be challenging because of its vague signs. However, the presence of predis-posing factors like obesity, gestation, lactation or gastrointestinal disease should raise clinical suspicion (Hughes et al, 2004).
The diagnosis of hyperlipaemia is confirmed by the measurement of triglyceride concentration within the blood. Healthy horses have triglyceride concentrations below 1.1 mmol/litre. If the concentration increases to between 1.1 mmol/litre and 5.5 mmol/litre, a diagnosis of hyperlipidaemia is made. Although this is usually not of significant clinical concern, it should still be monitored and it is recommended that veterinarians search for the cause of the negative energy balance to treat the underlying disorder and to start increasing caloric intake to prevent hyperlipidaemia from becoming hyperlipaemia (Hughes et al, 2004; Dunkel et al, 2014). When triglyceride concentrations exceed 5.5 mmol/litre, hyperlipaemia is diagnosed, and at this point, clinical signs and hepatic lipidosis are often observed (Watson et al, 1992b). There is no consensus about the prognostic value of triglyceride concentrations; however, in one study, none of the horses with triglyceride concentrations above 13.6 mmol/litre survived (Watson et al, 1992a; Mogg and Palmer, 1995; Dunkel et al, 2014). Monitoring the trend in triglyceride concentrations might be more important in establishing a prognosis, particularly when treatment is implemented (Mogg and Palmer, 1995; Oikawa et al, 2006).
Careful monitoring of circulating triglyceride concentrations in high-risk patients is therefore paramount (Table 3), and reliable stall-side analysers have been developed for horses (Naylor and Durward-Akhurst, 2012; Pongratz et al, 2016). Beyond triglyceride concentrations, horses with hyperlipaemia often develop metabolic acidosis, azotaemia and increased liver enzyme activity (aspartate amino-transferase and gamma-glutamyl transpeptidase) (Murray, 1985; Watson et al, 1992a; 1993; Moore et al, 1994; Hughes et al, 2002; Dunkel and McKenzie, 2003; Dunkel et al, 2014). Since the ketone pathway is poorly developed in horses, ketonaemia and ketonuria are not reported (Hughes et al, 2004).
Table 3. Haematological and biochemical variables requiring monitoring in the management of hyperlipaemia
| TriglyceridespHCreatinineUreaAspartate aminotransferaseGamma-glutamyl transpeptidaseAmmoniumAlbuminNeutrophil count |
Liver biopsies can also be used to confirm a diagnosis and determine the degree of hepatic lipidosis (Dunkel et al, 2014). Extremely vacuolated hepatocytes resembling adipocytes are common, and in severe cases, kidneys can also be infiltrated with lipids (Murray, 1985; Dunkel et al, 2014).
Management
Since the prognosis for hyperlipaemia is poor, successful management requires prompt identification of patients at risk and monitoring of triglyceride concentrations before clinical signs develop (Watson et al, 1993; Dunkel and McKenzie, 2003). This proactive approach is especially recommended for ponies, donkeys and miniature breeds (Mogg and Palmer, 1995; McKenzie, 2011).
The successful management of hyperlipaemia is based on two approaches: limiting the predisposing factor and addressing the negative energy balance. Correction of predisposing factors can be difficult or even impossible. Nevertheless, identification and treatment of any underlying disease can be enough to resolve an episode of hyperlipaemia. In severe cases where mares are pregnant or lactating, caesarean section or early weaning should be considered (Gay et al, 1978; Dunkel et al, 2014). Supportive care with fluid therapy to correct azotaemia and electrolyte and acid–base abnormalities is always recommended (Murray, 1985; Hughes et al, 2004).
Determination of daily energy requirements is challenging in practice. In a healthy mature horse, the daily energy requirements are estimated to be around 140 kJ/kg/day (33 kcal/kg/day), but they increase in sick horses and decrease with hospital confinement (Pagan and Hintz, 1986; Cruz et al, 2006; National Research Council, 2007). Overall, the daily energy requirement of a sick horse is considered to be around 95 kJ/kg/day (22 kcal/kg/day) (McKenzie, 2011).
Enteral nutrition is the first line of treatment if the gastrointestinal tract is functional. If spontaneous intake cannot meet the daily energy requirements, offering different types of palatable feed or adding molasses to feed to make it more palatable and encourage a more regular eating of a fibre-containing diet can be successful. Alternatively, administering small amounts of carbohydrate solution (such as 60 ml of corn syrup or molasses every 2 hours) can provide up to 21 kJ/kg/day (5 kcal/kg/day) (Watson et al, 1993; McKenzie, 2011). Although this is much lower than the recommended daily energy requirement, it could be enough to stimulate insulin secretion and limit lipid mobilisation (Watson et al, 1993; McKenzie, 2011). If the horse or pony has some degree of tissue insulin resistance, blood glucose levels should also be monitored to detect hyperglycaemia. If more energy is required, nasogastric intubation or placement of an indwelling enteral feeding tube might be considered (Golenz et al, 1992; Toth et al, 2014). The use of frequent nasogastric intubations allows the addition of a more fibrous diet but can be traumatic after a few days. Commercial enteric formulations delivered through an enteral feeding tube have been used to reverse hyperlipaemia (Golenz et al, 1992; Hughes et al, 2004).
In cases where enteral feeding is not possible (for example as a result of gastric reflux or ileus), parenteral nutrition is indicated (Moore et al, 1994; Durham et al, 2004). For 24 hours, a 5% dextrose solution at 5 ml/kg/h provides about 21 kJ/kg/day (5 kcal/kg/day) and can be enough to stimulate insulin secretion, inhibit lipolysis and decrease circulating triglyceride levels (Murray, 1985; Dunkel and McKenzie, 2003; McKenzie, 2011). Blood glucose should be monitored, and hyperglycaemia addressed with reduction of the fluid rate or insulin therapy (Murray, 1985; Hughes et al, 2004). If used, insulin can be administered subcutaneously or as a continuous rate infusion. Subcutaneous injection should start at 0.15 IU/kg, twice a day with 0.05 IU/kg increments if hyperglycaemia is not controlled, keeping in mind that delayed hypoglycaemia can occur. For continuous rate infusions, the starting dose is 0.07 IU/kg/h and blood glucose should also be monitored at least hourly until blood glucose has stabilised to ensure euglycaemia (4–8 mmol/litre). If adjustments are required, insulin infusion can be increased progressively while continuing to monitor blood glucose levels. If insulin and glucose are administered at the same time, only one infusion rate should be modified at a time (McKenzie, 2011).
If parenteral nutrition is considered for more than 24 hours, a partial parenteral nutrition solution with amino acids and carbohydrates should be used (Murray, 1985; McKenzie, 2011). Partial parenteral nutrition solutions can provide 63 kJ/kg/day (15 kcal/kg/day) and represent about 70% of the estimated daily energy requirement (Durham, 2006). Again, hyperglycaemia is a common complication of partial parenteral nutrition solutions but decreases in circulation triglyceride concentrations tend to be rapid after treatment initiation (Durham, 2006). When the partial parenteral nutrition is discontinued, transition to enteral nutrition is a critical step, and profound hypoglycaemia is a common complication. Reduction by 25% every 6 hours, and monitoring of blood glucose are warranted (McKenzie, 2011).
Heparin has been used for the management of hyperlipaemia as it promotes lipoprotein lipase activity and triglyceride use by peripheral tissues (Watson et al, 1992a). A dose ranging from 40–100 IU/kg, administered either intravenously or subcutaneously, once or every 8 hours can be used but results have been inconsistent (Watson et al, 1992a; Mogg and Palmer, 1995).
Prevention
In practice, the nutrition of sick or hospitalised patients is often overlooked. However, it is crucial to pay close attention to their actual dietary intake, especially in high-risk cases (Dunkel and McKenzie, 2003; Dunkel et al, 2014). In these cases, regular measurement of circulating triglyceride concentration is recommended. A study on miniature horses presented for colic showed that 70% of cases had increased triglyceride concentrations, suggesting that nutritional support might need to be considered before hyperlipaemia develops (Hughes et al, 2003). Long-term management plans to reduce obesity have been reviewed previously and should be implemented (Witherow, 2021).
Other lipid disorders
A syndrome resembling hyperlipaemia has been described in sick neonatal foals; however, the magnitude of circulating triglyceride concentrations is usually milder (4.3 mmol/litre) (Myers et al, 2009; Berryhill et al, 2017). These cases are often associated with sepsis and in one study, foals with circulating triglycerides concentrations above 2.3 mmol/litre were less likely to survive (Myers et al, 2009; Berryhill et al, 2017).
This disorder has a different pathogenesis from the syndrome described in adult horses, ponies and donkeys, as in addition to a negative energy balance, foals have some degree of primary liver disease after sepsis or neonatal isoerythrolysis leading to the lipid disorder (Tan et al, 2005; Myers et al, 2009).
Management is similar to that of adults with treatment of the primary condition and caloric support. The estimated daily energy requirement in sick neonatal foals is around 210 kJ/kg/day (50 kcal/kg/day) (Ousey et al, 1996; Jose-Cunilleras et al, 2012).
Conclusions
Disorders of lipid metabolism in adult horses, ponies and donkeys as well as neonatal foals can be life-threatening. Understanding the mechanisms of lipid metabolism and the factors that contribute to negative energy balance is essential for early detection and successful management of these conditions. Hyperlipaemia, characterised by high triglyceride concentrations and opalescent serum, requires proactive monitoring and treatment. Prevention through attentive nutritional support, especially in high-risk cases, can play a vital role in avoiding the onset of clinical hyperlipaemia. By addressing these challenges and implementing proactive measures, practitioners can improve the prognosis of lipid-associated conditions.
KEY POINTS
- Lipid disorders are common in ponies, donkeys and miniature horse breeds.
- A diagnosis of hyperlipaemia relies on the measurement of circulating triglyceride levels.
- Frequent monitoring of circulating triglyceride levels in high-risk cases is crucial to prevent hyperlipaemia.
- Stressors, such as transport or hospitalisation, can precipitate hyperlipaemia in high-risk patients.
- Management of hyperlipaemia relies on early detection, limiting the predisposing factors and addressing the negative energy balance.
- A similar syndrome is recognised in neonatal sick foals.


