Maggot debridement therapy utilises the natural life cycle of the larvae of the greenbottle fly (Lucilia sericata) to debride and digest devitalised and necrotic tissue.
Few clinicians in veterinary practice will have avoided the evocative sight of a maggot-infested wound, and while many only associate larvae with negative outcomes there is great potential in equine use.
Maggots as therapy
Medical maggots are the same species as those responsible for fly strike, but they are produced to be clinically sterile for use in hospitals and outpatient settings (Hinshaw, 2000).
Free range medical maggots used to be the only option for use, until the development of the ‘BioBag’, which allows the larvae to debride while ‘contained’ at the wound bed.
The BioBag is now accepted as the standard mode of application in human healthcare; the application and removal of which is far closer to that of a standard dressing (Figure 1).

A compromise between surgery and autolytic (natural) debridement, the beneficial aspects of larval therapy are threefold: debridement, disinfection and positive healing effects (Sherman, 2014).
Maggot debridement therapy
Maggot debridement therapy is a method of wound debridement that lies between surgical and autolytic techniques, as a combination of micro-mechanical, enzymatic, and autolytic debridement (Figure 2). Maggot debridement therapy has been found to achieve results faster through chemical and physical effects, compared to established methods of autolytic debridement using hydrogels (Mudge et al, 2014).
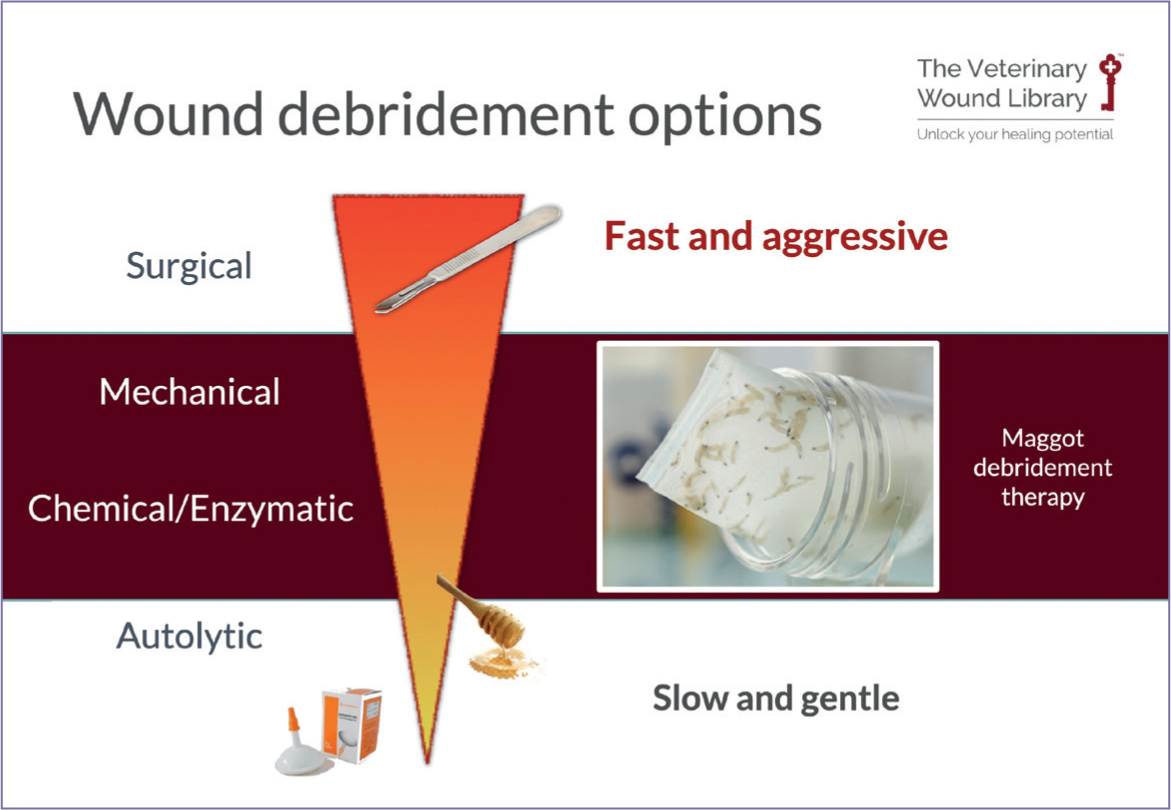
Wound debridement is critical to wound progression; a wound that retains slough, necrotic tissue, foreign bodies or debris and devitalised tissue will continue to produce mediators and enzymes, such as the matrix metalloproteinases that help dissolve proteins while inhibiting cell proliferation, wound contraction and epithelialisation.
While debridement is a key stage of the wound bed preparation process, the challenge in equines is that debridement cannot always be achieved surgically, often as a result of associated practical or economic challenges. An example of these cases is wounds or abscesses that penetrate anatomical structures such as the hoof capsule or spinal processes. Alternative methods of debridement may be more destructive, risk serious complication and are costly to perform.
The mode of action of maggot debridement therapy is based upon the feeding process of larvae that secrete a wide range of proteolytic enzymes to liquify proteins around them into a material that can be easily digested (Naik and Harding, 2017).
Physical movement of the larvae makes an active contribution to their ability to break down tissue. Using mouth hooks to grip onto tissue, the maggots pull themselves along the wound bed, into pockets and cavities, while small spines on their bodies abrade the tissue around them, allowing their enzymatic secretions to have maximal effect.
Disinfection
In the clinical setting, the antimicrobial effect of maggots is now well accepted as both physical and chemical. A combination of enzymatic secretions help to convert their environment into a more hospitable zone for feeding and growth, while the physical and chemical process of digestion means that they both consume bacteria, as well as excreting alkaline ammonia and other compounds into the wound bed that further inhibit bacterial proliferation. (Anderson et al, 2010)
Biofilms, which contribute to significant delays in wound healing, are also believed to be disrupted through the physical action of larvae. This combination of antimicrobial elements and physical disruption is credited with the ability to break down stubborn biofilms that may not respond to systemic or topical antibiotics. As such, maggot debridement therapy has become a somewhat novel addition to the armoury of the wider antimicrobial stewardship campaign.
Wound healing
Research isolating specific compounds that are exclusively beneficial for healing is currently unclear. Many compounds have been suggested, but it is not clear whether positive results have been gained from improved rates of debridement and decontamination, or as a result of unrelated mechanisms.
Allantoin, a substance excreted by larvae, has long been suggested to have a positive influence on wound healing. Commonly used in the cosmetic industry (from plant sources), allantoin has been associated with improved rates of granulation tissue formation in the past, but this assumption is dated. In reality, it may be a combination of both physical and compound factors that support fibroblast activity and stimulate angiogenesis.
Medical maggots: where are they from and how are they used?
The key difference between wild and medical maggots is that professional use requires sterile standards to be met, so medical maggots used in the UK will have been carefully reared in a specialist unit, most likely by the company BioMonde in Wales.
The process begins with the parent flies who are nurtured in dedicated housing and fed on a vegetarian diet to avoid the risk of microbial or viral contamination passing to their offspring, which may in turn harm the patient.
Each greenbottle fly will lay between 200 and 400 eggs a day when they are in full production, these then are carefully harvested by a team who transfer them to the hatchery. Once they are 2–3mm in size, they are ready to be safely sterilised and placed in groups inside their BioBag. The size of the bag is relative to the size of the wound it is intended for, with the appropriate number of larvae inside to debride the area beneath.
It is possible to store larvae for a day or two in the fridge when they arrive, but it is not recommended to leave them for long as they may become dry over time, which can affect their viability and mean the dressing may not perform as well as expected.
The BioBag is applied to the wound after normal preparation; clipping of surrounding hair, thorough lavage and removal of any loose debris and slough.
The dressing can be directly applied to the wound or packed inside a pocket or cavity, noting that one of the only contraindications historically associated with free-range maggot use is in wounds that connect to the abdominal cavity.
Where pocketing wounds or limbs are already producing copious exudate, it may be wise to use a barrier cream around the wound, such as Cavilon (3M), to protect the surrounding skin from excoriation.
On the typical 3-day duration of wear, the larvae will each produce enough enzymes to reduce 75g of protein to liquid. This fuels their growth and helps to produce the beneficial enzymes that promote granulation tissue formation.
Once they are 3 days old, the larvae will have achieved their maximum potential for debridement and should be disposed of via clinical waste. If the wound requires further debridement, a new bag can be applied for another 3 days until the wound is granulating.
Maggot therapy in equines
The use of maggot therapy for wound debridement is nothing new; its increasing adoption in human healthcare is based upon the clinical and economic benefits they offer, including selective and rapid debridement of complex wounds without the need for surgery. In patients who are unable to tolerate anaesthesia, where surgery is complex, or the wound heavily contaminated, it offers a practical solution for use in outpatient units. In cases where an equine is unsuitable for general anaesthesia, or where cost constraints require the patient to be managed at home, this could be similarly reflected in equine practice.
Cases supporting positive outcomes using maggot debridement therapy are positive and plentiful, especially as a rationale for the debridement of injuries to the hoof (Figure 3; Hinshaw, 2000; Lepage et al, 2012). Yet, it remains a modality that few are familiar with in general practice.
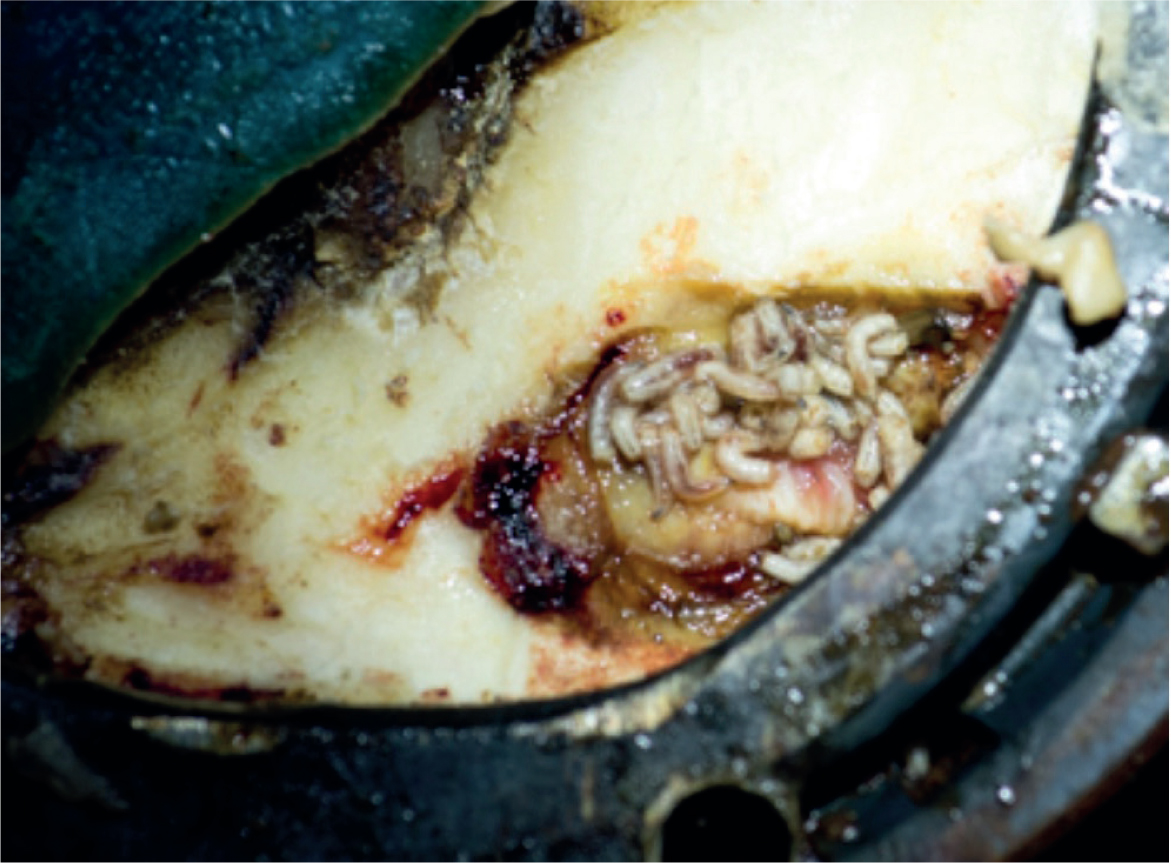
Indications include any wounds where debridement is required, with the particular benefit of being able to access areas where effective debridement is challenging, such as behind the hoof wall, or in areas where vital anatomical structures are present.
The highest risk in equine limb wounds and hoof injuries are to the larvae themselves. Bandaging and dressings may need to be creative to ensure the larvae are not squashed as they grow; the use of a felt or hydrocolloid ‘frame’ around the wounds may solve this problem. In the case of the plantar aspect of the hoof, the use of a hospital plate is recommended. When using the BioBag, it will initially appear flat and see through, but by day 3 the dressing will appear full and ‘puffed up’ with the larvae clearly visible after having increased in size almost 10-fold.
Conclusions
Use of maggot therapy is limited to date, likely owing to a lack of awareness of the benefits or expertise in applying them. However, this is a modality that should be more seriously explored for use in equines.
KEY POINTS
- Maggot debridement therapy offers an alternative to sharp debridement.
- The mode of action is through both physical disruption and enzymatic degradation of devitalised tissue.
- Maggot therapy can be used in areas that are hard to access by surgical means.
- Maggot therapy has already been used in the management of hoof injuries and penetrating wounds with great success.
To be part of the discussion, scan this QR code to complete a short survey on the use of maggot therapy and access a free webinar on the use of maggot therapy in equines, hosted by the Veterinary Wound Library.



