Johnson & Johnson Animal Health organised an intensive roundtable discussion on the topic of preventing surgical site infectios (SSIs). Panel members included eight senior clinicians from small animal and equine practice and both veterinary surgeons and registered veterinary nurses were represented. Challenges associated with SSI prevention and the value of care bundles were discussed.
A SSI is a healthcare-associated infection occurring at or near the site of surgery, up to 30 days after surgery or up to 1 year if implants have been left in place (Horan et al, 2008). SSIs are further classified as superficial incisional, deep incisional, or organ/space infections; diagnostic criteria are outlined in Table 1.
Table 1. Surgical site infection (SSI) criteria
| Type of SSI | Must meet the following criteria: | And at least one of the following criteria: |
|---|---|---|
| Superficial incisional |
|
|
| Deep incisional |
|
|
| Organ/space |
|
|
Adapted from National Healthcare Safety Network (2021)
SSIs result in additional patient morbidity and mortality (Jenks et al, 2014), as well as significant additional human healthcare costs (Piednoir et al, 2021). In veterinary medicine, the estimated incidence of SSIs is between 0.8 and 18.1% for small animal surgery (Nelson, 2011) and between 5 and 30% for equine surgery (Ruple-Czerniak et al, 2013). The large variation in incidence rates can be attributed to variation in surgical procedures, surgical sites, patient populations, and an absence of a standardised surveillance methodology (Weese, 2008). In human medicine, SSIs are estimated to cost US $21 to US $34000 per case (Iskandar et al, 2019; Monahan et al, 2020). A single-centre retrospective study quantified the cost of SSIs after tibial plateau levelling osteotomy to be between CA $125 to CA $5022 per patient (Nicoll et al, 2014). Another study showed a 74.4% increase in total costs in the event of an SSI (Espinel-Rupérez, 2019).
In addition to the tangible financial impact, SSIs have intangible effects on the client, the veterinary team and the veterinary practice. From the client's perspective, SSIs increase caregiver stress and may result in dissatisfaction with the veterinary surgeon and team. With regards to the veterinary team and practice, SSIs may affect staff morale, increase their workload, and affect practice reputation (Ethicon, 2022).
Broadly speaking, strategies to prevent SSIs can be stratified into preoperative, intraoperative and postoperative measures.
Preoperative measures
Preoperative measures include careful patient selection, preparation of the surgical team, hair removal, preparation of the surgical site and rational antibiotic prophylaxis.
Patients with concurrent endocrinopathies are 8.2 times more likely to develop an SSI (Nicholson et al, 2002), and the risk–benefit of elective surgery should be carefully weighed before proceeding. Recommendations are similar in human surgery (Mangram et al, 1999), although it must be recognised that endocrine disease is not a monolithic entity and each patient's condition needs to be considered individually.
Staff involved in surgery should keep their nails shorter than 2 mm to minimise bacterial load at the fingertips (Hardy et al, 2017; Anderson et al, 2021). The permissibility of nail polish is controversial; these same studies failed to find an association between wearing nail polish and bacterial counts (Hardy et al, 2017; Anderson et al, 2021). Prior to aseptic preparation (Figure 1), hands and nails should be washed to remove gross debris. Brushes are no longer recommended and do not offer any benefits over soap alone (Loeb et al, 1997). Asepsis of hands can be achieved with an alcohol-based hand rub (ABHR) or a medicated soap (Widmer et al, 2010). If using the latter, chlorhexidine has been shown to be superior to povidone iodine (Jarral et al, 2011). An ABHR should have an alcohol concentration of at least 80% to be maximally efficacious (Zandiyeh and Roshanaei, 2015). ABHRs offer certain advantages over soaps, in that they are quicker to apply, gentler on the skin, and avoid the possibility of post-scrubbing contamination from tap water (Leaper et al, 2008). The World Health Organization also recognises that ABHRs can increase compliance with hand hygiene, and recommends their use over soap (World Health Organization, 2009).
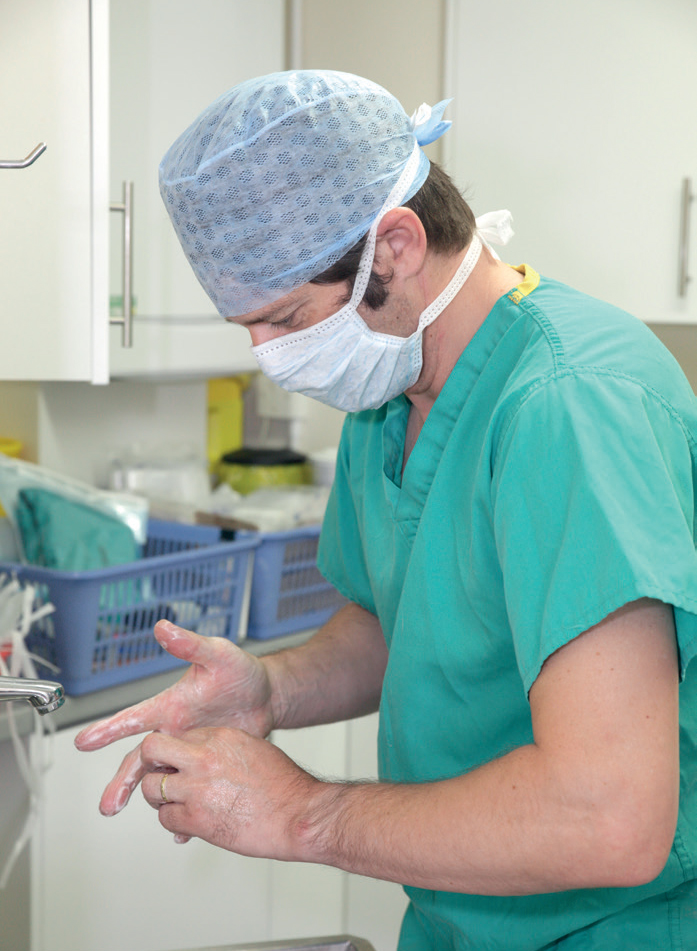
Clipping before anaesthetic induction has been shown to increase the likelihood of SSI (Brown et al, 1997) and hair removal should hence be performed after anaesthetic induction. Use of a razor has been associated with increased risk of SSI as a result of microtrauma to the skin (Bowers, 2012); clean, well-serviced clippers and clipper blades should be used instead. Clipper blades should be cleaned and disinfected between patients to minimise fomite transmission of pathogens.
Prior to surgical skin preparation, scrubbing should be carried out to remove gross debris with either a chlorhexidine or povidone iodine-based detergent (Bowers, 2012). The site is then surgically prepped after moving to the sterile theatre. An alcohol-based chlorhexidine solution is recommended provided there are no contraindications to its use (NICE, 2020). A 2% solution of chlorhexidine should be used, as a minimum (Hasegawa et al, 2022), and this concentration adhered to regardless of the size of the patient. A survey of veterinary practices showed inconsistent training and awareness of the correct method of surgical skin preparation among staff and this remains an area for improvement (Evans et al, 2009; Ethicon, 2022).
Prophylactic antibiotics are indicated in certain surgeries to reduce the risk of SSI developing. The Centers for Disease Control (CDC) classifies surgical wounds as clean, clean-contaminated, contaminated or dirty (CDC, 1991). Guidelines for antibiotic use in surgery, adapted to small or large animals, are available from professional bodies (British Equine Veterinary Association (BEVA), 2022; British Small Animal Veterinary Association (BSAVA), 2022). Prophylactic antibiotics are not necessary in clean procedures, unless an implant is placed, the patient is compromised and at high risk of SSI, or the consequences of SSI would be catastrophic (Nelson, 2011). Prophylactic antibiotics may be used in clean-contaminated or contaminated procedures. The choice of antibiotic should be based on the anticipated pathogens, which varies according to location of surgical procedure (Verwilghen and Singh, 2015). Antibiotics should be administered intravenously at least 30 minutes before the first incision such that tissue concentrations are sufficient, and repeating every 60–90 minutes throughout surgery (Sathoo et al, 2021). The prolonged use of antibiotics in the pre- and postoperative periods is generally not recommended (Berríos-Torres et al, 2017).
Intraoperative measures
Intraoperative measures include theatre wear (Figure 2), patient draping, anaesthetic management, surgical principles, surgical and anaesthetic time, wound closure with triclosan-coated sutures, wound closure patterns, and wound dressings.
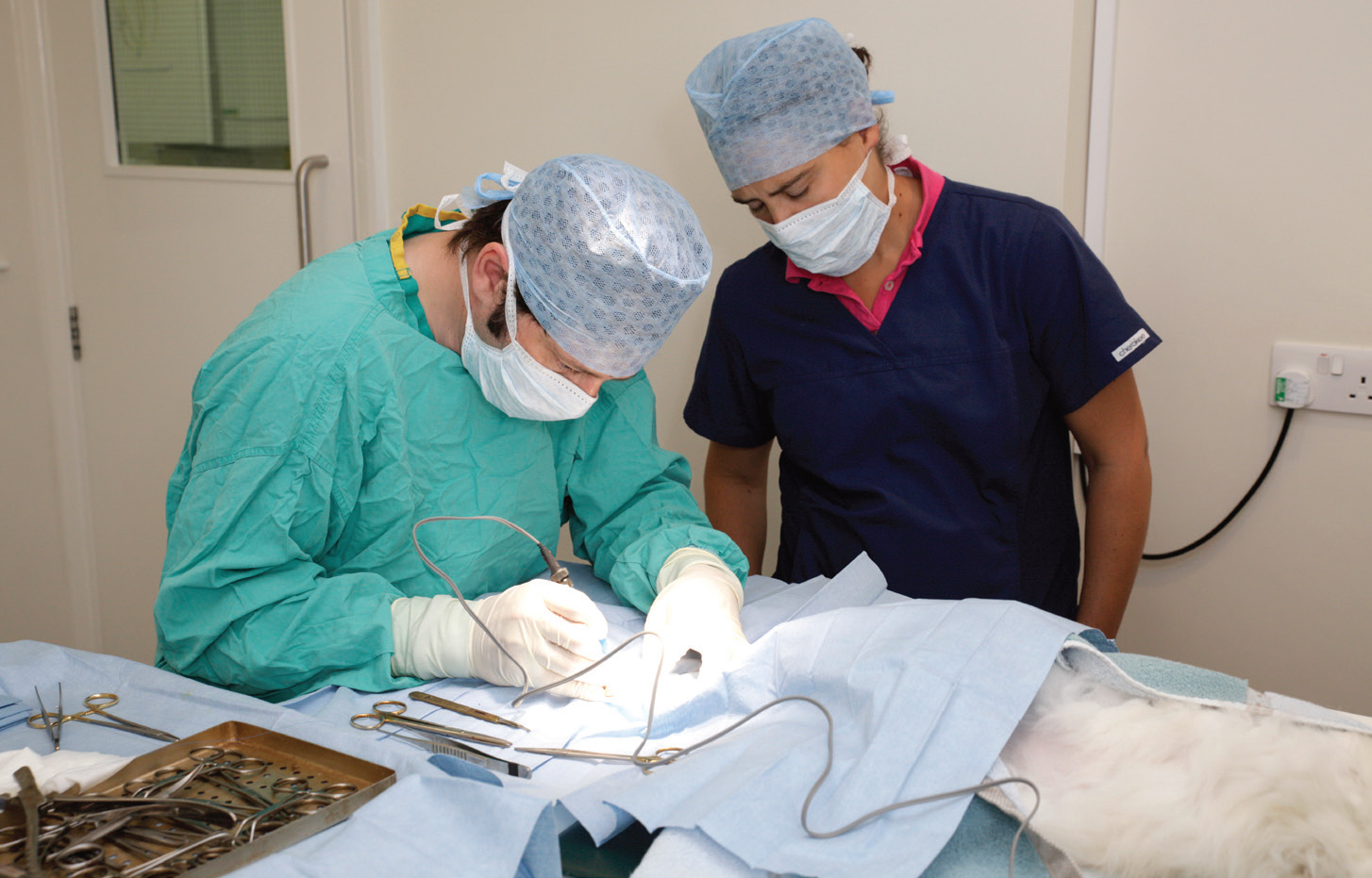
Theatre staff are traditionally dressed in a set of clean scrubs and theatre shoes, not worn in other areas of the hospital. Evidence that this attire significantly impacts SSI rates is lacking, however the differentiated attire can promote a sense of theatre discipline among staff and indirectly contribute in other ways (Verwilghen and Singh, 2015). Face masks are also traditionally worn, although systematic reviews have failed to show a difference in SSI rates between masked and unmasked surgeries (Aikabeli, 2021). Reusable cloth skullcaps have been shown to have greater impermeability and less microbial shed than disposable skullcaps and bouffant caps (Markel et al, 2017).
The use of disposable versus reusable drapes is a controversial topic. There is a body of evidence to suggest that disposable drapes are superior in preventing SSIs (McHugh et al, 2014; Showalter et al, 2014). Recent evidence is challenging this belief (McQuerry et al, 2021) and it has been suggested that reusable drapes may be acceptable for clean surgeries, provided laundering protocols are adhered to strictly (Vasanthakumar, 2019). The routine use of incise drapes is not recommended as their use may increase the risk of SSIs (Falk-Brynhildsen et al, 2013; NICE, 2020; Webster and Alghamdi, 2015).
If used, iodophor-impregnated incise drapes are recommended over plain incise drapes (NICE, 2020). For surgeries where the distal limb is included in the sterile field, wrapping the foot with an impermeable layer such as a piece of surgical drape is essential to prevent bacterial strikethrough (Vince et al, 2008).
Strict adherence to Halsted's principles is recommended not only to minimise the risk of SSIs, but also to improve other outcomes of surgery (Figure 3). These include adequate haemostasis, aseptic technique, gentle handling of tissues, preservation of blood supply, minimisation of tissue tension, accurate approximation of tissues and minimisation of deadspace. It is challenging, if not impossible, to conduct clinical trials to investigate the relationship between surgical technique and SSI rates. Surgeon experience, used as a proxy for this, has been shown to be correlated with the risk of SSI (Torfs et al, 2010; Wormstrand et al, 2014).
Anaesthetic protocols should also be optimised to minimise the development of SSIs. Hypotension and hypoxaemia increase the risk of SSIs (Costa-Farré et al, 2014; Turk et al, 2014), likely as a result of reduced tissue perfusion and alterations in local immune function. Time under anaesthetic has also been shown to be a risk factor, with longer surgeries more likely to result in SSI (Beal et al, 2000; Eugster et al, 2004; Thieman Mankin and Cohen, 2020; Rigby et al, 2021). However, it must be recognised that anaesthetic time may not be an independent risk factor as this variable can be associated with procedure type and surgical time. Perioperative hypothermia has been demonstrated to be a significant risk factor in the development of SSIs in humans (Berríos-Torres et al, 2017; Bu et al, 2019), in contrast with veterinary medicine where the effect of hypothermia on SSI rates is unclear (Beal et al, 2000; Espinel-Rupérez et al, 2019). It is prudent to maintain patient normothermia for other reasons such as cardiovascular stability and patient comfort. Good quality evidence is available in human medicine regarding perioperative glycaemic control, optimisation of tissue oxygenation and maintenance of normothermia. Further veterinary-specific studies are necessary.
Wound closure with triclosan-coated sutures is recommended by the CDC, NICE and the WHO (Berríos-Torres et al, 2017; NICE, 2020; World Health Organization, 2018). Despite their increased costs, the use of these suture materials is likely to result in an overall reduction in costs because of downstream effects (NICE, 2020). Triclosan is a broad-spectrum antimicrobial with both Gram-positive and Gramnegative activity (Jones et al, 2000) and has been shown to have in vitro and in vivo antibacterial activity when applied to suture material (Ming et al, 2008). Meta-analyses in human surgery have shown overwhelming evidence in favour of their use (Daoud et al, 2014; Edmiston et al, 2014; Ahmed et al, 2019). The evidence base in veterinary surgery is conflicting: in vitro evidence of efficacy (McCagherty et al, 2020) has not been demonstrated in vivo (Bischofberger et al, 2010; Etter et al, 2013; Thieman Mankin and Cohen, 2020). Monofilament is recommended over multifilament suture material, which may harbour bacteria and predispose to development of SSI (Costa-Farré et al, 2014).
The choice of suture pattern in wound closure is largely dictated by surgeon preference and experience. Available studies suggest that, in both small animal and equine surgery, the use of subcuticular or intradermal patterns may lead to fewer complications (Colbath et al, 2014; Zellner et al, 2016; Scharner et al, 2018). The evidence is mixed regarding the use of staples for skin closure (Verwilghen and Singh, 2015), and a study comparing intradermal poliglecaprone 25 closure versus staples did not demonstrate a difference in SSI rates (Atwood et al, 2015).
Once wound closure is complete, a dressing may be applied to the surgical wound. In human surgery, it is unclear if a dressing or a type of dressing is superior in reducing the risk of SSIs in primarily closed surgical wounds (Dumville et al, 2016). A veterinary study showed no difference between covered and uncovered surgical wounds after tibial plateau levelling osteotomy (TPLO) surgery (Aktay and Giannetto, 2019). Antimicrobial dressings, such as those containing elemental silver or manuka honey, are available but require further clinical evaluation (O'Dwyer, 2013). In equine surgery, the use of an abdominal bandage or a stent bandage can be considered to reduce the risk of incisional complications (Smith et al, 2007; Tnibar et al, 2013).
Postoperative measures
As mentioned earlier, the CDC recommends restricting antimicrobial use to the perioperative period. However, numerous veterinary studies have demonstrated a protective effect of postoperative antimicrobial therapy in a select population of patients undergoing TPLO (Fitzpatrick and Solano, 2010; Frey et al, 2010; Nazarali et al, 2014; Solano et al, 2015) and its use can be considered in a select population of patients.
Surveillance
Surveillance programmes are widely used in human surgery not only to establish the true incidence of SSIs, but also to direct action to drive down the rate of SSIs (Brandt et al, 2006; Rioux et al, 2007). Broadly speaking, surveillance is the systematic collection of data in relation to a particular patient population, procedure, or event (Burgess, 2019). Active surveillance involves collecting primary data at predetermined timepoints, such as via client telephone follow-ups. Active surveillance is costly and time consuming, but is better able to accurately estimate the true incidence of SSIs in a given population (Burgess, 2019). Passive surveillance, on the other hand, relies on collecting secondary data such as microbiological results from a laboratory, or rates of patient re-presentation post-surgery. Passive surveillance is cheaper and quicker to implement, provided that practice management systems collecting these data are already in place; however, this method of surveillance is likely to underestimate the true incidence of SSIs (Burgess, 2019). Veterinary registries collecting data for specific patient populations and procedures exist (International Colic Audit, 2019; Canine Cruciate Registry, 2021). Large-scale surveillance systems are lacking in veterinary surgery. The available veterinary literature is in agreement with the human literature (Turk et al, 2014; Garcia Stickney and Thieman Mankin, 2017), in that active surveillance programmes identified a significant number of SSIs that would otherwise have been missed.
Care bundles
A care bundle is a collection of evidence-based interventions delivered together to improve patient care and outcomes (McCarron, 2011; Lavallée, 2017), compared with if the interventions were delivered individually. Care bundles have origins in intensive care medicine and are widely used by emergency medicine and critical care practitioners (Horner and Bellamy, 2012). The use of care bundles has expanded and they are now widely used in other disciplines such as obstetrics and gynaecology, palliative care and respiratory medicine (Hopkinson et al, 2011; Meredith and Evans, 2014; Morton and Morris, 2020). Care bundles have been applied in the area of SSIs and have shown significant reductions in SSI rates as a result (Crolla et al, 2012; van der Slegt et al, 2013).
Care bundles may be developed at all levels of healthcare delivery, from the international level to the local level. For example, the Surviving Sepsis Campaign was initiated at a conference level among intensive care practitioners and has since expanded internationally (Levy et al, 2003). Care bundles can be simply developed at the local practice level or alternatively, developed through collaboration among subject matter experts, academia, industry associations and industrial partners (Ethicon, 2022). A care bundle approach has been used in veterinary orthopaedic surgery with positive results (Stine et al, 2018). Care bundles are not meant to be applied as rigid frameworks or meant to restrict clinician freedom (Ethicon, 2022). How to develop care bundles is beyond the scope of this paper and the reader is referred to other resources (Borgert et al, 2017).
Potential challenges and human factors
Staff training and compliance with hand hygiene and other standard operating protocols to prevent SSIs are hugely important in preventing SSIs. Time pressure and lack of training resulting in suboptimal compliance is recognised in veterinary practice (Anderson et al, 2013; Ethicon, 2022). The panel discussion identified a lack of compliance, training and time as three major barriers to implementing infection prevention strategies in veterinary practice (Figure 4). Identifying the systemic barriers and human factors contributing to suboptimal compliance is important in approaching SSI prevention in a holistic manner (Musuuza et al, 2019).
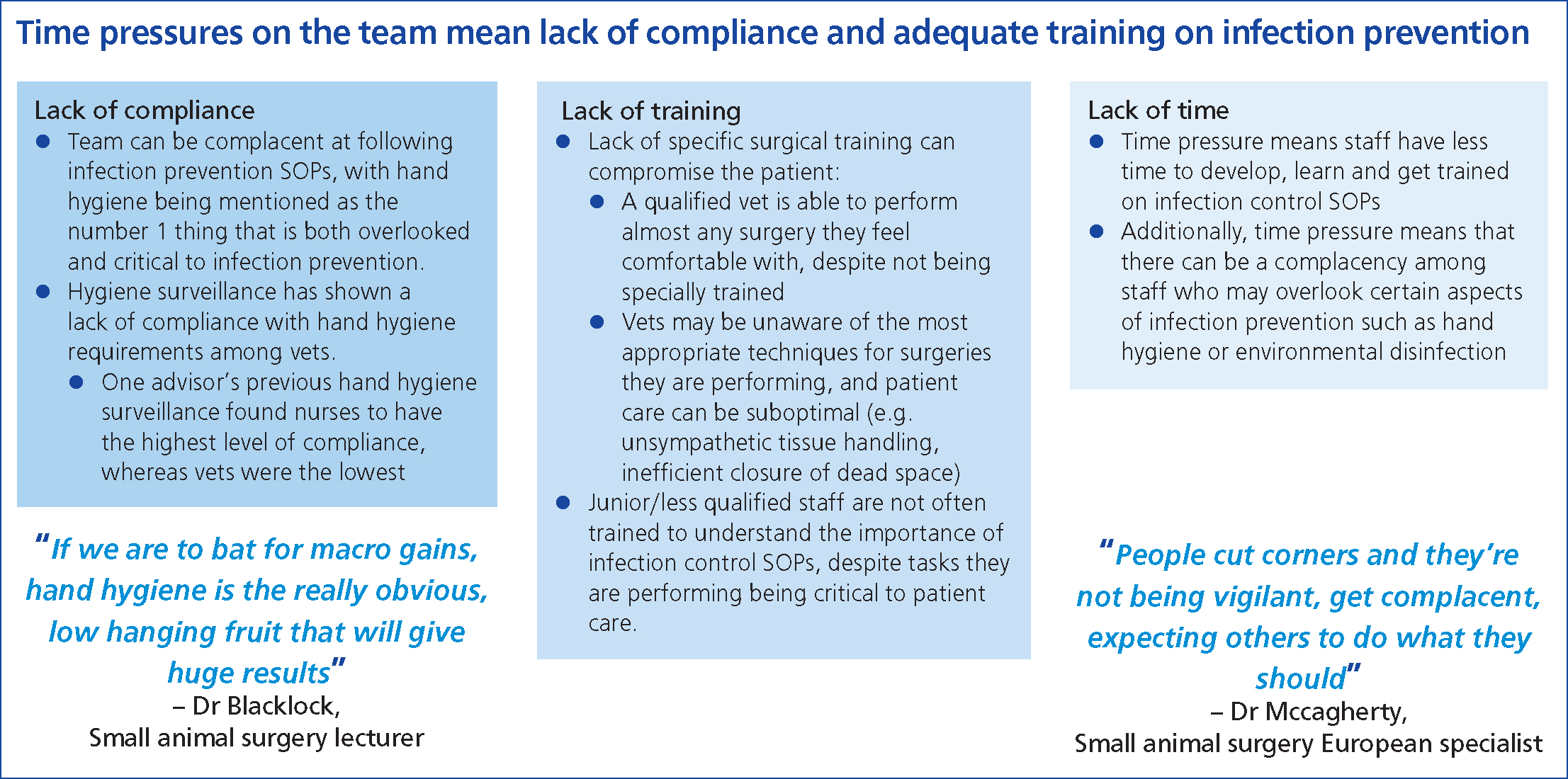
It is increasingly recognised that team culture can significantly affect the perioperative management of the patient and hence, the SSI risk (Pettis, 2018). A proactive team culture must be cultivated and maintained. Each team member must be proactive and empowered, and every patient encounter must be driven by evidence-based practice. A zero tolerance approach to SSIs must be adopted, and team members must remain open-minded as new evidence is generated. With such a multidisciplinary approach involving the entire veterinary team, marginal gains can be achieved and the incidence of SSIs in veterinary practice can be further driven downwards (Ethicon, 2022).
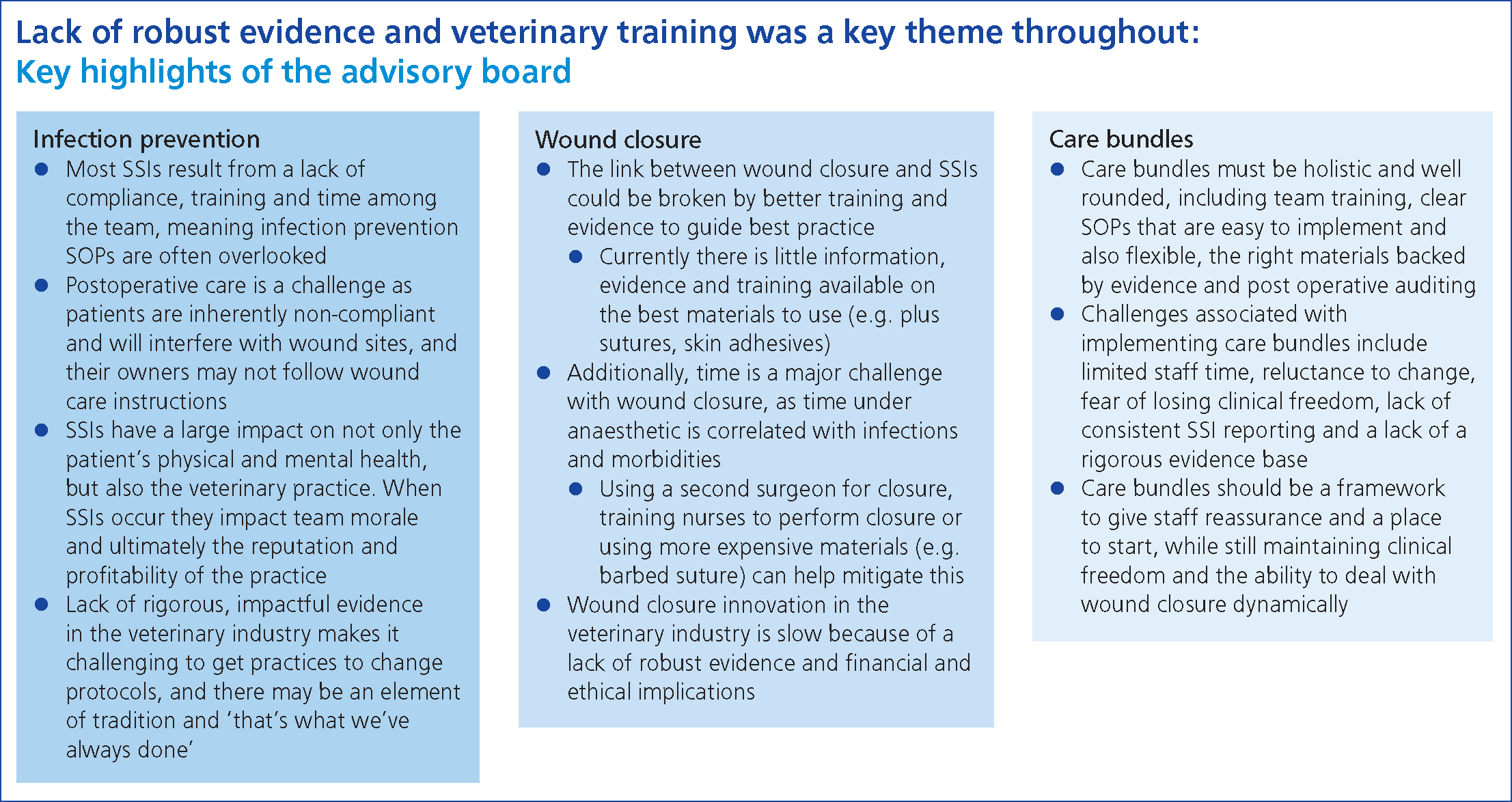
Key highlights from the advisory board
One of the key highlights from the advisory board held by Ethicon was the lack of robust evidence and the lack of training for veterinary professionals (Ethicon, 2022). The key highlights of the meeting are illustrated in Figures 5 and 6. Communication within practice during surgical procedures is essential (Ebeck, 2022) — the members of the advisory board recognised the importance of creating a culture in which staff feel they can voice opinions, and the impact this would have on improving infection prevention. In particular they recognised that: ‘staff need to be able to speak up when they have made a mistake, if a team member is not following protocol correctly or if they do not feel adequately trained.’ In addition, record keeping was deemed essential by the advisory board: ‘reviewing theatre checklists regularly, recording patient movement through the hospital and using online/app-based checklists can improve infection prevention.’
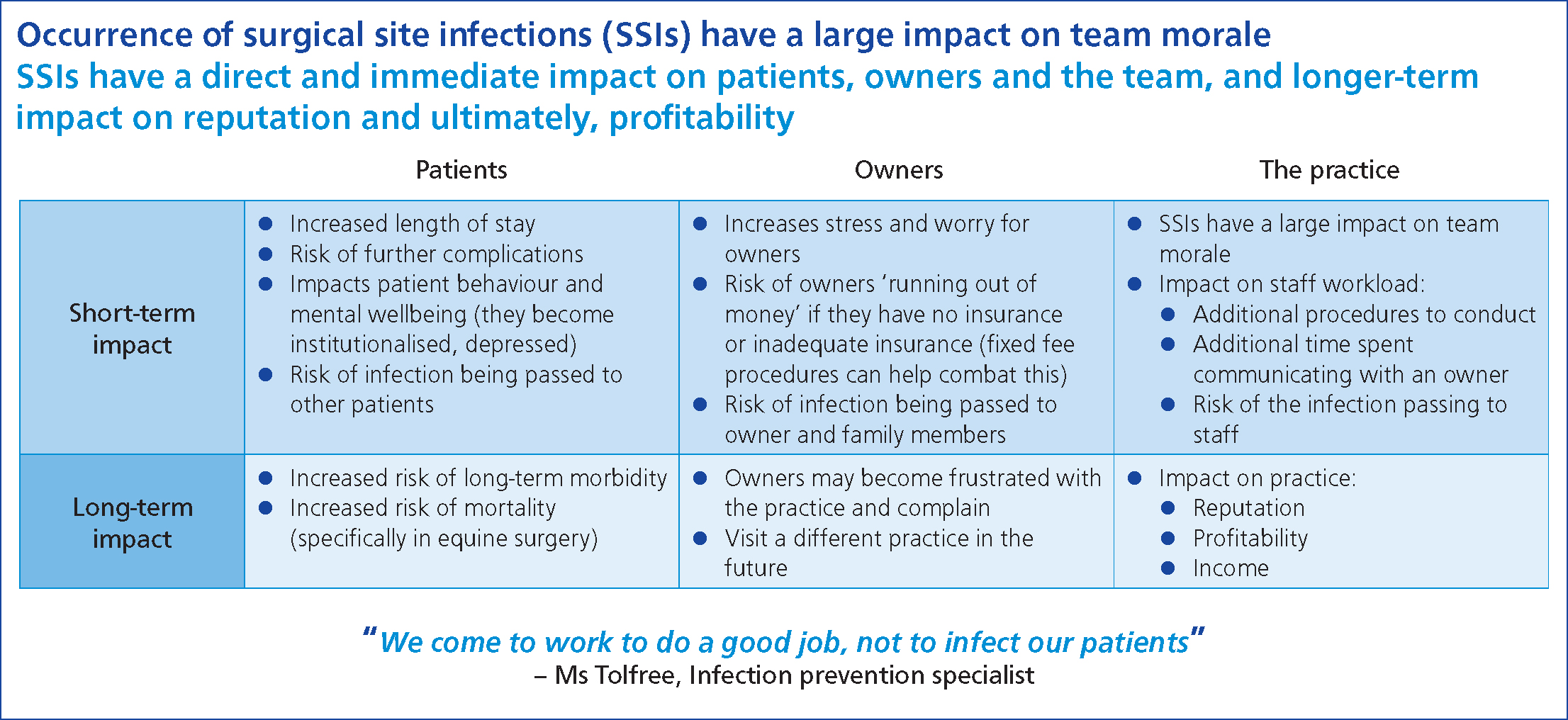
One important finding of the advisory board was the impact of SSIs on staff morale (Figure 6). It was recognised that SSIs have a direct and immediate impact on patients, owners and the veterinary team, and potentially longer-term impacts on the practice's reputation and, ultimately, profitability.
Conclusions
There remain significant knowledge gaps in veterinary surgery. The veterinary evidence base is far smaller and the studies of lower quality in comparison to that available in the human literature. Lack of a standardised definition of SSIs and a widely applicable surveillance system is a major limit with regards to the epidemiology of SSIs in veterinary surgery. Care bundles can provide a framework for veterinary teams to implement evidence-based interventions, with the aim of incrementally improving clinical outcomes. Practitioners, industry and academia must collaborate to create high-quality bundles which will be widely applicable and acceptable. A well-trained, highly-motivated veterinary team is essential in preventing SSIs, and the importance of these human factors should not be overlooked.
KEY POINTS
- Surgical site infections (SSIs) have an impact on the patient, the client and the veterinary team.
- SSI prevention begins preoperatively and continues into the postoperative period.
- Accurate surveillance techniques are needed to appreciate the true extent of SSIs in a population of patients.
- Lack of compliance, training and time are key human factors limiting outcomes.
- There is a lack of robust veterinary evidence. Practitioners, academia and industry should collaborate further.


